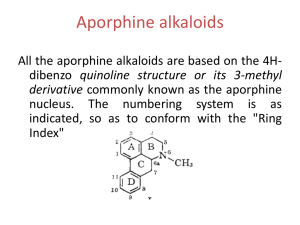
Biosynthesis some alkaloids - Organic Chemistry
advertisement

Biosynthesis some alkaloids Piperine indole Isolasi and Identifikasi Indole Alkaloids • Occurrence: 1- Ergot Alkaloids Ergot is the dried sclerotium of a fungus, Claviceps purpurea (Fam. Hypocreacea) that arise on the ovaries of the rye plant (Secale cereale, Fam. Gramineae). • Consumption of flour contaminated with Ergot led to many serious intoxications known as (Ergotism- Ignis Fire) in Europe. • Ergot can be detected in flour by using UV light where contaminated flour will show violet spots. • Classification of Ergot Alkaloids: A- Clavine Type Alkaloids: Simple water soluble bases with little medicinal value. All end with “clavine: e.g. Agroclavine. B- Lysergic acid Amides: They are all derivatives of (l)-Lysergic acid and subclassified into: 1- Simple lysergic acid amides: Composed of Lysergic acid and simple amines. 2- Polypeptide Alkaloids: Composed of Lysergic acid and at least 3 amino acids. • General Characters: Ergot alkaloids are N-monosubstituted amide derivatives of both lysergic acid and its isomer isolysergic acid that differ only in configuration at C-8. On treatment with ammonia lysergic and isolysergic acids give the corresponding amides ergine and isoergine respectively. COOH COOH 8 N CONH 2 N CH3 CH3 N CH3 10 N H N H Lysergic acid Isolysergic acid N H Ergine Members related to lysergic acid (e.g. ergotamine and ergometrine) are levorotatory, more active and designated by suffix “ine”. Members related to isolysergic acid (e.g. ergotaminine and ergometrinine), are dextrorotatory, less active and designated by suffix “inine”. 1- Simple Lysergic acid amides • Characters: 1- Composed of Lysergic acid and simple amines. 2- Low molecular weight. 3- Water Soluble. Ergonovine (Ergometrine) OH H 2C Composed of (l)-lysergic acid and 2-aminopropanol. Its (d) isomer is called Ergometrinine. CH3 HC HN CO 8 N CH3 10 • Uses: It causes vigorous contraction of the uterus. It is mainly used as an oxytocic in order to aid delivery or to prevent postpartum hemorrhage. N H = = Ergonovine (l) (Ergometrine) Ergonovinine (d) (Ergometrinine) Lysergic acid diethylamide (LSD) • It is a semisynthetic product. • LSD has potent CNS stimulant effect. • LSD is one of the abused drugs. C2H5 CON C2H5 8 N 10 N H CH3 2- Polypeptide Alkaloids • Characters: They are derivatives of Lysergic acid with a complex polypeptides of at least 3 amino acids. They have high molecular weight. They are insoluble in water. This class include medicinally important members. Ergotamine • Characters: Its (d) isomer is called Ergotaminine. The peptide moiety is composed of 3 amino acids: a-Hydroxyalanine Proline Phenylalanine OH O CH3 N • Uses: Treatment of migraine as it constricts the peripheral blood vessels. Has some oxytocic (ecobolic) activity. O CO N CH2 O 8 N 9 10 N H CH3 Structure Activity Relationship: • Lysergic acid must be in the (l) form. The (d) isomers are inactive. • Saturation of the 9- 10 double bond of Ergotamine gives Dihydroergotamine, a compound with antimigraine effect but no oxytocic effect. OH O CH3 N O CO N CH2 O 8 N 9 10 N H CH3 Stability: • The active (l) form convert to the (d) isomer by the effect of Alkalis or prolonged storage in alcohol. HO O C O C R R C H R H 8 8 N N CH 3 10 10 8 CH 3 N CH 3 10 (l) form (d) form • Addition of water to the 9- 10 double bond takes place in aqueous acidic solutions, upon exposure to day or UV light. The resulted Lumi alkaloids are inactive. O C R H 8 HO 10 N CH3 • Tests for identification: Van-Urk's Reagent (p-dimethyl aminobenzaldehyde (PDAB) in 15% H2SO4, containing traces of FeCl3) + Alkaloid → Deep blue color. Erlich Reagent (p-dimethyl aminobenzaldehyde (PDAB) in H2SO4) + Alkaloid → Deep blue colour. Keller’s test: Solution of the alkaloids in acetic acid with traces of FeCl3 + concentrated H2SO4 on the wall of the test tube → blue layer is formed between the two phases. 2- Vinca (Catharanthus) Alkaloids Occurrence: Catharanthus or Vinca is the dried whole plant of Catharanthus roseus G. Don (or Vinca rosea L), Fam. Apocynaceae. It contains about 150 alkaloids, the most important are vinblastine and vincristine. • Classification: 1- Monomeric Alkaloids: These are alkaloids that contain either indole or indoline: Indole monomers e.g. Catharanthine Indoline monomers e.g. Vindoline and Vincamine. 2- Dimeric Alkaloids: Homogenic dimmers: Composed of two indole or indoline monomers. Mixed dimmers: One indole and one indoline monomers e.g. Vincristine and Vinblastine. 1- Monomeric Alkaloids: H N H3COOC OH N N N N H H3COOC C2H5 Vincamine C2H5 N CH 3 H3COOC Catharanthine COOCH 3 OH Vindoline • Vincamine Enhances the cerebral blood flow, facilitate cerebral circulation metabolism and increase general activity. Vincamine is used in cerebral vascular deficiency and atherosclerosis in elderly patients. 2- Dimeric Alkaloids: Mixed dimmers • These are dimeric alkaloids having indole and indoline (dihydro-indole) nuclei e.g. Vinblastine and Vincristine • Vinblastine and Vincristine They occur in very minute amounts in Vinca (0.003- 0.005); 500 Kg of the plant yield only 1 gm of vincristine. They are very important for cancer treatment. Vincristine is more active but isolated in smaller amounts than Vinblastine. Vinblastine can be converted to vincristine chemically or by microbial transformation using Streptomyces albogriseolu . Structures: • Vinblastine (Vincaleukoblastine) is produced by coupling of Catharanthine and Vindoline. • Vincristine (leurocristine) has CHO istead of CH3 in the vindoline part of Vinblastine. N N H H3COOC R=CH3 Vinblastine R=CHO Vincristine HO N N R H3COOC COOCH 3 OH • Tests for identification: 1-Vanillin /HCl reagent gives with: Vinblastine a pink color. Vincristine an orange-yellow color. 2-Van-Urk's reagent: → Reddish-brown color. • Uses : Vinblastine is used for treatment of Hodgkin's disease (Pseudoleukemia or Lymphatic anaemia) and carcinoma resistant to other therapy. Vincristine has a cytotoxic effect .It is useful in the treatment of leukemia in children, small cell lung cancer, cervical and vaginal cancers. • Mechanism: Both alkaloids are Antimetabolites interfere with the syntheses of Desoxyribonucleic acids. Semisynthetic derivatives: N • Vindesine: N H H3COOC HO It is used for treatment of acute lymphoid N leukemia in children. N COOCH 3 CH 3 H2NOC OH N • Vinorelbine: N H H3COOC HO It is an oral anticancer with broader activity and lower neurotoxicity than vinblastine. N N CH 3 H3COOC COOCH 3 OH 3- Physostigma (Calabar beans) Alkaloids • Source: Physostigma venenosum. • Constituents: Physostigmine (Eserine). • Properties: It is a tertiary base, possessing an ester linkage. Contains 3 Nitrogen atoms. Eserine on alkaline hydrolysis Eseroline + Methylamine + CO2 O O M e -HN HO Me Alkaline Hydrolyses N N N H Me Eserine Me Me N H Me Eseroline + CH3NH2 + CO2 Me Eserine upon oxidation, in presence of trace of alkali e.g. NaOH, or traces of metals is transformed to rubreserine (red compound). Therefore, it could be affected by the alkalinity of glass containers during storage. HO O Me oxidation Me O N N H N Me Me Eseroline N H Me Me Rubreserine • Tests for identification: Eserine blue test: Eserine + strong ammonia solution → Yellowish red color , evaporation → Bluish residue soluble in alcohol (eserine blue). Eserine + alkali hydroxides → Red color. • Uses: It has a cholinergic effect and stimulates glands’ secretion (c.f. atropine) act by inhibition of choline-esterase. So used in poisoning by Solanaceous Alkaloids. Treatment of Alzheimer’s disease due to cholinergic effect . Eserine is mainly used as a myotic drug in the treatment of glaucoma. Diaphoretic in cases of kidney dysfunction. 4- Nux-Vomica Alkaloids • Source: – Seeds of Strychnose nux vomica and Ignatus beans. • Constituents: 5% Alkaloids mainly Strychnine and Brucine. • Properties: Brucine is the dimethoxy derivative of Strychnine. Both alkaloids contains 2 Nitrogen atoms. Hemitoxiferine is a degradation product of strychnine. Dimerization of hemitoxiferine produces a valuable skeletal muscle relaxant Toxiferine. N R R N O O R= H Strychnine R= OCH3 Brucine • Tests for identification: • Nitric acid test: Drops of concentrated nitric + few crystals of the alkaloids: Strychnine gives a faint yellow color that on evaporation turns to yellow color Brucine gives an intense red color, that on evaporation and addition of SnCl2 solution turns to violet. • Tests for strychnine: Sulfuric acid-dichromate test: Few crystals of strychnine + drops concentrated H2SO4 + few crystals of K2Cr2O7 → deep blue streaks → violet → purplish red → orange → yellow. Test with Mandalin's reagent: Strychnine gives Deep violet blue color, add water → red → cherry-red. • Uses: Strychnine is extremely toxic. It is used in veterinary medicine as CNS stimulant and tonic. It is used as antidote in barbiturate poisoning. It is also used as rodenticide. Brucine is less toxic than strychnine. It is sometimes used as CNS stimulant, Commercially it is used as alcohol and oil denaturant Vincristine Overview • I. Physical Properties • II. Synthesis • III. Characterization • IV. History and Uses • V. How it works General Information2 • • • • • CAS Number: 57-22-7 Molecular Formula: C46H56N4O10 Molecular Weight: 824.97 Melting Point: 218-220°C Clear liquid (taken by injection) Synthesis from a Natural Source • Vincristine has two natural sources: Catharanthus roseus (Vinca rosea), the Madagascar periwinkle, and Tabernaemontana pachysiphon • This yield from the Madagascar periwinkle is less than 1μg/g (.0003%)6 Synthesis from vinblastine H2CrO4 Ethyl Acetate 2 hours, -83- -25°C 80-90% yield5 • This is the easiest and most common method of synthesis, as well as one can give high yields Synthetic Production Discovered in 2004 First route to make synthetic vincristine6 Spectroscopy • Mass Spectrum4: M+1=825 • UV Spectrum1: 220, 255, 296 nm • IR Spectrum6: 3445, 2924, 2852, 1740, 1681, 1597, 1498, 1458, 1369, 1230, 1033, 747 cm-1 History and Uses • Discovered in 1953 by Robert L. Noble, and cleared by the FDA in 1963 as Oncovin, marketed by Eli Lilly and Co. • Used to treat acute leukemias and all lymphomas • Side effects include nervous system damage and constipation • Work is being done to develop more effective derivatives with fewer side effects8 How it works • Stops division of cells • Enters cell during mitosis and blocks formation of microtubules of the mitotic spindle during metaphase3,7 Bibliography • • • • • • • • (1) Anonymous Vincristine. In The Merck index: an encyclopedia of chemicals, drugs, and biologicals; O'Neil, M. J., Ed.; Merck: Whitehouse Station, NJ, 2006; pp 17171718. (2) Anonymous Chemfinder.com Database and Internet Searching. http://chemfinder.cambridgesoft.com (accessed Jan. 22, 2007). (3) Anonymous Overview: The Mitotic Spindle. http://www.sinauer.com/pdf/nspcellcycle-6-0.pdf (accessed Apr. 15, 2007). (4) Choi, Y. H.; Kim, J.; Yoo, K. Supercritical Fluid Extraction and Liquid Chromatography-Electrospray Mass Analysis of Vinblastine from Catharanthus roseus. Chem. Pharm. Bull. 2002, 50, 1294--1296. (5) Huhtikangas, A.; Laakso, I.; Seppaenen-Laakso, T.; Verma, A. Patent Application Country: Application: FI; Patent Country: FI Patent 107924, 2001. (6) Kuboyama, T.; Yokoshima, S.; Tokuyama, H.; Fukuyama, T. Stereocontrolled total synthesis of (+)-vincristine. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 11966-11970. (7) Lurie, P. M.; Manaster, J.; Stryckmans, P. A.; Vamecq, G. Mode of Action of Chemotherapy in vivo on Human Acute Leukemia--II. Vincristine. Europ. J. Cancer 1973, 9, 613--620. (8) Szabo, L.; Bolcskei, H.; Eszter, B.; Mak, M.; Szantay, C. Synthesis of vinca alkaloids and related compounds, Part XCV: Nitration study of vinblastine-type bisindole alkaloids. Arch. Pharm. Pharm. Med. Chem. 2001, 334, 339--405. Quinoline Quinoline Alkaloids Cinchona Alkaloids • Cinchona bark contain many alkaloids the majors are: 1- Quinine and Quinidine. HO COOH 2- Cinchonine and Cinchonidine. • Cinchona alkaloids are present as salts with Quinic and HO OH Cinchotannic acids. OH Quinic acid • They are diacidic bases form two types of salts: 1- Neutral salts (Monoacidic) (less water soluble. 2- Acidic salts (Diacidic) water soluble. • Both Quinine and Quinidine, Cinchonine and Cinchonidine are Diastereoisomers. Each stereochemistry at C-8 and C-9. HO H3CO N H3CO Quinine in 8 N H N differs HO 9 H pair H H N Quinidine the Properties: • Quinine is very slightly soluble in H2O, soluble in ethanol, chloroform, ether, benzene and other organic solvents. • Quinine is a diacidic base. It forms 2 types of sulfates: – Quinine monosulfate (neutral and H2O insoluble). – Quinine bisulfate (acidic and H2O soluble). • Quinine (l-isomer) gives quinidine (d-isomer) among other products when warmed with KOH in amyl alcohol. • Quinine is levorotatory, while quinidine is dextrorotatory. Separation of the 4 Alkaloids Powdered Bark - Alk. CaO + NaOH + H2O - Reflux with benzene & - Filtration while hot Benzene filtrate (Alkaloidal bases) dilute H2SO4 Acidic aqueous layer (Alkaloidal bisulphates) pH to 6.5 with Na2CO3 Precipitate (monosulphate) Quinine Hot water, Na2CO3 Aqueous solution (Monosulphates) quinidine, cinchonine & cinchonidine NaOH, ether Quinine Ether layer Quinidine & Cinchonidine Aqueous layer -HCl PH=7, Na, K Tart. salt - filter Precipitate Filtrate Cinchonidine tartrate Quinidine tartrate Cinchonine • Tests for identification 1- Fluorescence test: Solution of the alkaloid in oxygenated acids (e.g H2SO4, HNO3 or phosphoric acid) blue fluorescence (+ ve with quinine and quinidine). 2- Thalleoquine test: Aqueous solution of the alkaloidal salt + Br2 /H2O (few drops till the appearance of yellow color) + NH4OH emerald green color (+ ve with quinine and quinidine). 3- Rosequin test (Erythroquinine test): Aqueous solution of the alkaloidal salt + dil HCl + Br2 /H2O (few drops till the appearance of yellow color) + CHCl3 + pot. Ferrocyanide + NH4OH red color in the CHCl3 layer (+ ve with quinine and quinidine). Uses: • Quinine is used mainly as anti-malarial in a dose of 2g of quinine sulfate or other salt for 14 days. • Quinidine is used as a cardiac depressant (anti-arrhythmic), particularly to inhibit auricular fibrillation in a dose of 0.6-1.6 g of quinidine sulfate daily. • Cinchonine and cinchonidine are used as antiinflammatory. Isoquinoline Alkaloids 1- Ipecacuanha Alkaloids • Occurrence: Ipecac is the dried roots and rhizomes of Cephalis ipecacuanha (Brazilian ipecacuanha) or Cephalis acuminata (Cartagena or Panama ipecacuanha) Fam. Rubiaceae. It contains several alkaloids (2 –2.5 %), mainly emetine (50- 70 % of total alkaloids), with cephaline and psychotrine. MeO RO MeO OMe N H OMe H N R = Me Emetine R=H Cephaline HO OMe OMe N H N Psychotrine • Emetine: It is non phenolic and levorotatory. It contains 2 basic nitrogens. • Cephaline: It is phenolic and levorotatory. It gives emetine on methylation with (CH3)2 SO4. • Psychotrine: Occurs as yellow prisms. It is phenolic and gives cephaline on reduction. It gives emetine on reduction followed by methylation. Isolation of Ipeca alkaloids: Powdered root and rhizome - Ext. alcohol,Conc., lead acetate. - Filtration Filtrate Alkaloidal bases and salts Residue Non-alkaloidal sub. - Evap., dil. HCl & Filtration Filtrate (Alkaloidal HCl salts) - Alk. with NaOH & Ext. with ether Ether layer Emetine Aq.alk. solution(Phenolic alkaloids) Aqueous layer (Psychotrine) - HCl (Alkaloidal salts) - Alk. NH4OH Ext. ether Ether layer (Cephaline) Tests for identification of Ipeca alkaloids: • Alkaloidal solution in HCl + Ca hypochlorite orange color. • Emetine and cephaline + Froehd's reagent: dirty green color (the color with emetine fades by addition of HCl). • Psychotrine + Froehd's reagent: pale green color. • Cephaline and psychotrine + p-nitrodiazobenzene dye soluble in NaOH purple color. • Psychotrine + conc. H2SO4 + HNO3 cherry red color. • Emetine + Liebermann's reagent black color. Uses: • Emetine and cephaeline have antitumour and antiviral activity, but are too toxic for therapeutic use. • Emetine and psychotrine are mainly used as emetic drugs. • The crude drug is used as expectorant (due to its saponin content) . • Emetine HCl and Dehydroemetine (DHE) have an antiamoebic effect, and are used for the treatment of amoebic dysentry and Fasciola. • Ipeca alkaloids are diaphoretic, alone or in combination with opium (e.g. Dover's powder). 2- Curare Alkaloids • Occurrence: • Curare or South American arrow poison is the dried crude extract, obtained mainly from Chondrodendron tomentosum (Menispermaceae) and certain Strychnos species, (Loganiaceae). • Curare contains several alkaloids (4-7%), the most important is d-tubocurarine. MeO N Me OH H O O Me Me H N+ OH OM e • Properties: d-tubocurarine (4ary alkaloid) is freely soluble in H2O. It is a phenolic dextrorotatory alkaloid. It is a bis-benzyltetrahydroisoquinoline alkaloid. • Tests: Saturated aqueous solution + FeCl3 faint green color green color. Solution of the alkaloidal HC1 + Na2CO 3 yellow brown precipitate. • Uses: Tubocurarine chloride is mainly used by i.m. or i.v. routes as skeletal muscle relaxant. It is used to control and reduce convulsions of strychnine poisoning and of tetanus. It is used as a diagnostic aid in myasthenia gravis.