PHG 322 lecture 1
advertisement

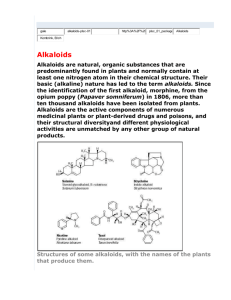
بسم هللا الرحمن الرحيم PHG 322 PHARMACOGONSY II LECTURE 1 PRESENTED BY ASSISTANT PROF. DR. EBTESAM ALSHEDDI Office: second flour # 84 Email: ebtesam.saad@yahoo.com Site: faculty.ksu.edu.sa/10252 Topics to be covered: (cont.) 1) Plants containing Alkaloids (botanical and chemical Introduction Phenylalkylamine, Tropolone and Imidazole alkaloids Pyridine and piperidine alkaloids Tropane alkaloids Quinoline and isoquinoline alkaloids Opium alkaloids Indole alkaloids Carboline, purine, steroidal and terpenoidal alkaloids 3 characters): Topics to be covered: Biomedical potential of marine natural products Microalgae as a drug source Biologically active substances from marine sponges 4 2) Marine derived drugs: Topics to be covered: 3) Plants containing Glycosides (botanical and chemical characters): Introduction Phenolic, cyanogenic and thioglycosides Coumarins, flavonoidal glycosides and related Anthracene derivatives Cardiac glycosides Saponins 5 compounds Topics to be covered: 4) Toxicological Pharmacognosy: Poisonous plants Plants as drugs of abuse Hepatotoxic plants Toxic mushrooms 6 3) Herb-drug interactions Important Book/Thesis: 1 2 3 Book/Thesis Editor or Author Pharmacognosy Trease 16th edition and Evans النباتات السامة في عبد هللا بن أحمد المملكة العربية السعودية األمير Bioactive Marine Bhakuni Natural Products and Rawat Date of pub. 2009 Name of Publisher Saunders London, New York, … جامعة الملك سعود 2000 كلية العلوم قسم النبات واألحياء الدقيقة 2005 Anamaya Publishers, New Delhi, India 7 No. The assessment tasks during the semester: Marks Assessment task Dr. Ebtesam Dr. Rababb First ½ of semester (Exam + Activity/Quiz) 18 - Second ½ of semester - 18 Final Exam Total 24 20 20 100 8 Practical Alkaloids Definition Alkaloids, which mean alkali-like substances, are basic nitrogenous compounds of plant or animal origin and generally possessing a marked physiological action on man or animals. • The nitrogen is usually contained in a heterocyclic ring system. Deviation from Definition 1) Some alkaloids are not basic e.g. Colchicine, Piperine, 2) Few alkaloids contain nitrogen in a non-ring system e.g. Ephedrine, Colchicine. Mescaline 3) Plant origin: Some alkaloids are derived from bacteria, fungi, insects, frogs, animals. Function of alkaloids in plants 1) Protective function against insects or herbivores 2) They are end products (detoxification) 3) Source for energy and reserve of nitrogen DISTRIBUTION IN PLANT: All Parts e.g. Datura. Barks e.g. Cinchona Seeds e.g. Nux vomica Roots e.g. Aconite Fruits e.g. Black pepper Leaves e.g. Tobacco Latex e.g. Opium Nomenclature Alkaloids terminate with the suffix: (ine) Genus name e.g. Atropine from Atropa Species name e.g. Cocaine from Coca Common name e.g. Ergotamine from Ergot Physiological activity e.g. Emetine (emetic) Discoverer e.g. Pelletierine from Pelletier 12 Their names may be derived from: Prefixes and suffixes These are, usually, added to the name of the parent alkaloid and are used to designate related alkaloids, generally present in the same plant. Examples are: I. Prefixes 1) Nor-: designates N-demethylation or N-demethoxylation e.g. Nornicotine 2) Apo-: designates dehydration e.g. Apomorphine 13 3) Iso-, Pseudo-, Neo- and Epi- indicate different types of isomers II. Suffixes 1) –dine: designates isomerism as in case of the Cinchona alkaloids quinine (l) & quinidine (d) cinchonidine (l) & cinchonine (d) 2) –ine: indicates, in case of ergot alkaloids, a lower pharmacological 14 activity e.g. ergotaminine is less potent than ergotamine Physical properties: 1) Condition: Most alkaloids are crystalline solids Some are liquids non-volatile volatile e.g. pilocarpine and hyoscine e.g. nicotine and coniine 2) Colour: Most alkaloids Some are coloured: - colchicine & berberine - canadine orange yellow 15 colourless 3) Solubility: Solubility of alkaloids & their salts is important because: a) They are often administered in solution b) Differences in solubility between alkaloids & their salts is used for: - isolation of alkaloids 16 - purification from non-alkaloidal substances Solvent Alkaloidal bases Alkaloidal salts Alcohol Soluble Soluble Soluble except: - morphine & psychotrine → insol. in ether - theobromine & theophylline → benzene (insol.) H2O Insoluble except: - caffeine - ephedrine - codeine - colchicine - pilocarpine - quat. ammonium bases Insoluble except: - lobeline hydrochloride → CHCl3 (soluble) Soluble except: quinine sulphate → insoluble in H2O 17 Organic solvents 4) Optical activity: Many alkaloids are optically active due to ? → presence of one or more asymmetric carbon atom(s) 18 • Optically active isomers may show different physiological activities. • l-ephedrine is 3.5 times more active than d-ephedrine. • l-ergotamine is 3-4 times more active than dergotamine. • d- Tubocurarine is more active than the corresponding l- form. • Quinine (l-form) is antimalarial and its d- isomer quinidine is antiarrythmic. • The racemic (optically inactive) dl-atropine is physiologically active. Chemical properties: Alkaloids contain: C, H, N Most of them contain oxygen Few alkaloids are oxygen free 19 nicotine coniine CHEMICAL PROPERTIES: I- Nitrogen: Primary amines R-NH2 e.g. Norephedrine Secondary amines R2-NH e.g. Ephedrine Tertiary amines R3-N e.g. Atropine Quaternary ammonium salts R4-N e.g d-Tubocurarine II- Basicity: R2-NH > R-NH2 > R3-N Saturated hexacyclic amines is more basic than aromatic amines. 1) Nitrogen in alkaloids Number of nitrogen atoms Type of amino group Basicity 1) Number of nitrogen atoms: • Usually one N atom • Some contain more than one → up to 5 N atoms e.g. nicotine → two ergotamine → five 21 2) Type of amino group: N in alkaloids → in form of amine • Primary amino group H Secondary amino group R R1 .. .. N H R2 N Tertiary amino group Quaternary ammonium ion R1 R1 H R2 ..N R3 R2 N + R4 R3 e.g. Norpseudoephedrine Ephedrine Nicotine Tubocurarine 3) Basicity: A lone pair of electrons on N atom → basic character → alkaloids resemble NH3 in chemical characters → form salts with acids without liberation of H2O N: Alkaloid + H+Cl- [ ] N H + Cl - Alkaloid hydrochloride 23 Factors influencing the degree of basicity: Structure of the molecule e.g. degree of unsaturation of heterocyclic ring N .. N .. Pyridine Piperidine Piperidine alkaloids → more basic than pyridine alkaloids unsaturation decreases basicity Presence & position of other substituents & fuctional groups Electron withdrawing gps e.g. carbonyl group decrease basicity 24 Electron releasing groups e.g. alkyl group increase basicity → Alkaloids may be: Neutral (not basic) → electron availability on nitrogen atom decreases → due to the presence of acidic group(s) e.g. alkaloids containing carboxylic group Narceine e.g. phenolic alkaloids Morphine 25 Amphoteric Ricinine