Lecture 16
advertisement
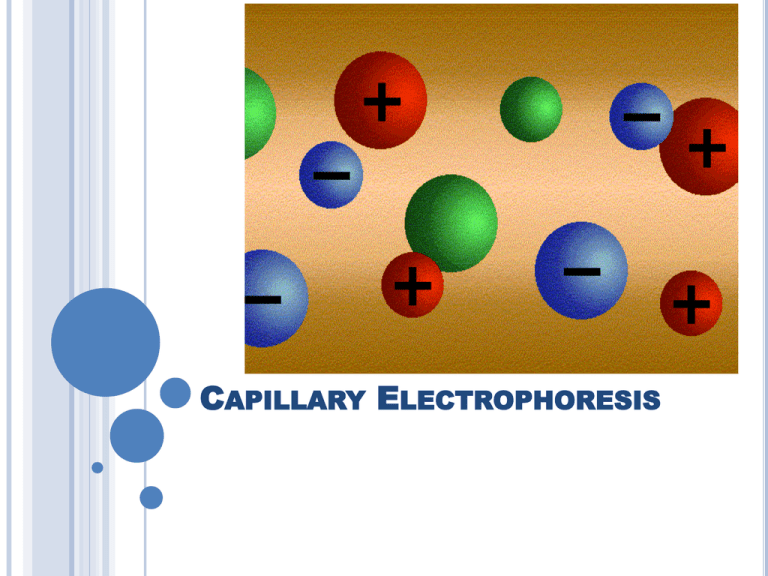
CAPILLARY ELECTROPHORESIS Electrophoresis is the movement of electrically charged particles through a media caused by an electric current. A high voltage, up to 30 kV is applied causing positively charged cations to migrate towards the negative electrode (cathode) and negatively charged anions to move toward the positive electrode (anode). Electrophoresis and Separation The main principle of separation is that small molecules move faster than large molecules, and molecules with higher charges move faster than ones with lower charges. Therefore, molecules are separated based on their charge/size ratios. There is another factor in capillary electrophoresis that contributes the movement of ions and produces additional separation ELECTROOSMOTIC FLOW ELECTROOSMOTIC FLOW When an electric field is applied, a layer of positive ions forms along the capillary walls. This mobile layer of cations is pulled towards the cathode. This movement of cations is great enough to drag the whole buffer system (bulk flow) along through the capillary tube. ELECTROOSMOTIC FLOW Normally, cations migrate towards the cathode, and anions towards the anode. However, the electroosmotic flow is great enough to move both positive and negatively charged molecules towards the cathode. This makes it possible to analyze both cations and anions in a single run. ELECTROOSMOTIC FLOW Under normal hydrodynamic pressure, friction along the wall of the capillaries causes the flow to slow down compared to the flow in the center of the capillary. This difference in flow velocities from the side wall to the center of the capillary causes band broadening. Electroosmotic flow is generated at the side walls of the capillary and because of this, the flow profile is much more uniform than in normal capillary flow. EFFICIENCY Plate Height, H van Deemter Plot H = A + B/u + (Cs + Cm) u A + B/u + Cu Mass Transfer (both), Cu Multipath Term, A Longitudinal diffusion, B/u Linear Velocity, u Plate Height, H EFFICIENCY X H = A + B/u + (Cs + Cm) u B/u + Cu Mass Transfer (both), Cu Longitudinal diffusion, B/u Linear Velocity, u Plate Height, H EFFICIENCY X X H = A + B/u + (Cs + Cm) u Therefore Capillary Electrophoresis is very efficient. Typical plate counts (N) for capillary electrophoresis are in the range of 100,000 – 200,000 compared to 5000 to 20,000 for B/u typical HPLC. Longitudinal diffusion, B/u Linear Velocity, u SAMPLE INJECTION METHODS Sample introduction is very important since extremely small samples are used (1 – 50 nanoliter or even less). These very small sample sizes make reproducible quantification of analytes difficult. There are advantages to using very small samples. For Example: The end of the capillary can be heated and pulled down to an extremely small diameter. These very fine points on the end of the capillary can be used to sample inside single cells or even within structures of the cell. SAMPLE INJECTION METHODS Hydrostatic Injection: immerse one end of the capillary in the sample container and either pressurizing the container or applying a vacuum to the other end of the capillary. Gravity Injection: the capillary is inserted into the sample vial and the sample vial is raised up above the level of the column outlet and the sample is siphoned into the column. The amount of sample introduced is based on time the capillary is placed in the sample. SAMPLE INJECTION METHODS Electrokenetic injection: One end of the capillary is inserted into the sample and voltage is applied across the column. The amount of sample taken up is based on the electrophoretic mobility of the sample ions, the electroosmotic flow, and differences in the ionic strength of the sample and buffer. This method can produce biases in sample introduction since mobile ions are taken up at a faster rate. However, electrokenetic injection can be used as a pre-concentration step and significantly lower the detection limits. Micellar Electrokinetic Capillary Chromatography When sodium dodecyl sulfate (SDS) is added to the buffer above a critical concentration, it forms aggregates called micelles. One end of the SDS molecule is a hydrophillic SO3 group while the rest of the molecule is a hydrophobic 12 carbon chain. The molecules group together with their hydrophobic ends together and their negative charges to the surface of the micelle. Micellar Electrokinetic Capillary Chromatography MEKC can be thought of as a combination of electrophoresis and reversed phase HPLC. The hydrophobic part of the micelle acts like the C-18 molecules in an HPLC column. A compound of intermediate water solubility will partition between the buffer and the hydrophobic parts of the micelle. Since the micelle moves slower than buffer, analytes that partition mostly in the buffer, will move relatively quickly while analyte molecules that partition mostly into the micellewill move more slowly. CHIRAL CHROMATOGRAPHY Enantiomers can be separated with capillary electrophoresis by the use of chiral additives to the mobile phase. Cyclodextrins are commonly used for this purpose. Cyclodextrins are rings of usually 6, 7, or 8 glucose molecules. Optical isomers associate significantly differently with the cyclodextrins and can be separated. Factors Affecting Separations Voltage- Generally produces sharper peaks and shortens analysis time (up to a point). Capillary Internal Diameter.- Better separations are achieved with smaller diameter. Small ID limits the amount of sample that can be used and the detector performance is lower. Buffer – Increasing the buffer pH will increase EOF. Increasing the buffer ionic strength will decrease EOF, but in many cases it will result in increased resolution. Use of different buffer ionic species can have a dramatic effect on resolution. Modifiers. Organic modifiers can be used to increase the solubility of many analytes. Organic modifiers such as methanol reduce flow while acetonitrile increases flow. ADVANTAGES OF CAPILLARY ELECTROPHORESIS • High Efficiency (N =100,000 to 1,000,000) • Short Analysis Times • Simultaneous Separation of Anions and Cations • Small Sample Volumes (pico- to nanoliters) • Low Consumption of Mobile Phase • Inexpensive Columns ($5 - $100) • Extremely Versatile