PPT
advertisement

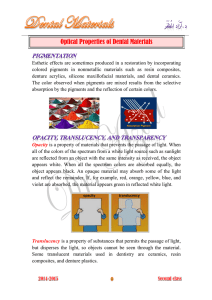
The Dual Nature of Light Wave and Particle Light as a particle Particles or packets of light are known as photons Brightness or intensity depends on the number of photons absorbed/unit area/unit time Photon carries fixed amount of energy Determines how fast it vibrates high energy = fast low energy = slow Light as a wave The distance moved by a photon during one of it vibrations is referred to as its wavelength abbreviations used in class: = lambda = wavelength unit = nanometers (nm) One nanometer = 10-9 meter = nu = my shorthand for light Long wavelength Low energy Short wavelength High energy Effective wavelengths for photosynthesis A prism separates visible light into a color spectrum The visible spectrum ROYGBIV Between 380 and 750 nm Why only visible light? Sunlight has three components 4% UV (ultraviolet radiation) TOO STRONG! ionizing radiation breaks weak chemical bonds causes DNA damage and sunburn absorbed by O2 and O3 (ozone), glass, plastic 52% IR (infrared radiation) TOO WEAK! low energy most energy of IR converted to heat 44% visible light JUST RIGHT! suitable energy for life - photosynthesis absorbed by pigments What Happens to Light? Reflected Chlorophyll is green because it reflects the green wavelengths. Sky is blue because water molecules reflect blue wavelengths. Transmitted Glass permits most visible wavelengths to pass through. Absorbed Chlorophyll absorbs all colors except green, especially red and blue regions. Pigments Molecules that absorb light Black absorbs all wavelengths White absorbs no wavelengths Color that we see is the reflected wavelengths Quality of Light Light quality (availability of different wave-lengths) can limit rate of photosynthesis Blue and red wavelengths are absorbed by chlorophyll Green wavelengths are reflected or transmitted Therefore most plants look green to us Usable wavelengths are called PAR – photosynthetically active radiation Chlorophyll Structure tetrapyrrolic rings Mg+2 atom phytol chain (tail) Forms chl a grass green absorbs in red and violet blue regions chl b, c, and d bluish green Chlorophyll Absorption spectrum: absorbs all wavelengths except green Absorbs most strongly in red and blue region of spectrum Action spectrum: doesn’t match absorption spectrum Chl a is primary photosynthetic pigment But there must be others Accessory Pigments Functions Extend range of photosynthesis by absorbing wavelengths not picked up by chl a Carotenoids also protect against photooxidation Oxygen radicals formed by excited electrons kill cells (basis for some herbicides) Accessory Pigment Types Chlorophylls Chl a is the main photosynthetic pigment Grassy green absorbs around 420 and 660 nm Chl b bluish green absorbs 453 and 642 nm about half as abundant as chl a Chl c and d Accessory Pigment Types Carotenoids fat soluble absorb 460 and 550 nm Blue, bluegreen, violet Structure - see plant chem. Notes Example: -carotene reddish yellow, orange precursor to vitamin A (split to get 2 vit A) needed to produce retinal pigment for vision carrots, tomatoes, bananas, squash, fall leaves Accessory Pigment Types Carotenoids are also found in animals egg yolks flamingo feathers squid ink corals fish amphibians lots of colors proteins attached to carotenoids remove protein => see red or orange this is what happens when you cook shrimp or lobster Absorption spectra Accessory Pigment Types Xanthophylls and fucoxanthin (brown algae) yellowish or red absorb blue light (some plants grow towards blue light) not as efficient as -carotene Phycoerythrin and phycocyanin found in cyanobacteria and red algae Remember: these bacteria as well as the algae contribute to production in aquatic systems Pigments Light reaching the surface of the earth Peak irradiation occurs at approx 500 nm Light intensity drops off at higher & lower wavelengths Pigments If we combine all of these, including chlorophyll and carotenoids, there is good light absorption across the entire visible spectrum! Absorption of Light by Water Absorption of Light by Water Red & blue wavelengths preferentially absorbed by water Wavelengths 500 – 600 nm absorbed least by water correspond with absorption spectra of phycobilins. Ecological Significance of Phycobilins Organisms with Phycobilin pigments are better adapted for life in an aquatic environment and can exploit greater depths in lakes and oceans! Therefore, red algae and the cyanobacteria can survive at greater depths than any other algae or vascular plants. Why Do Leaves Change Color in the Fall? Chlorophyll synthesis shuts down Chlorophyll molecules break down “True” colors of leaves show through Accessory pigments Carotenoids - orangeIn chromoplasts Xanthophylls - yellow Anthocyanins - red, purple In central vacuole Quantity of Light Amount of light reaching thyllakoid membranes is limited by Location of chloroplast in leaf Incident angle of sunlight as it strikes leaf Self shading by other leaves Shading by competitors See discussion of Chazdon 1985 and Tanaka et al. 2001. Global Light Availability Tropical latitudes - day and night equal Polar latitudes continuously light at midsummer, continuously dark at midwinter Maximum sunlight energy greater in tropics than polar regions Global Light Availability Maximum sunlight energy greater at high altitudes than at sea level Damaging UV-B radiation greater in tropics than polar regions, high elevations vs. low elevations Biochemical protection: Flavonoids to absorb UV-B Increased levels of antioxidant enzymes and DNA repair enzymes