Science of light lab
advertisement
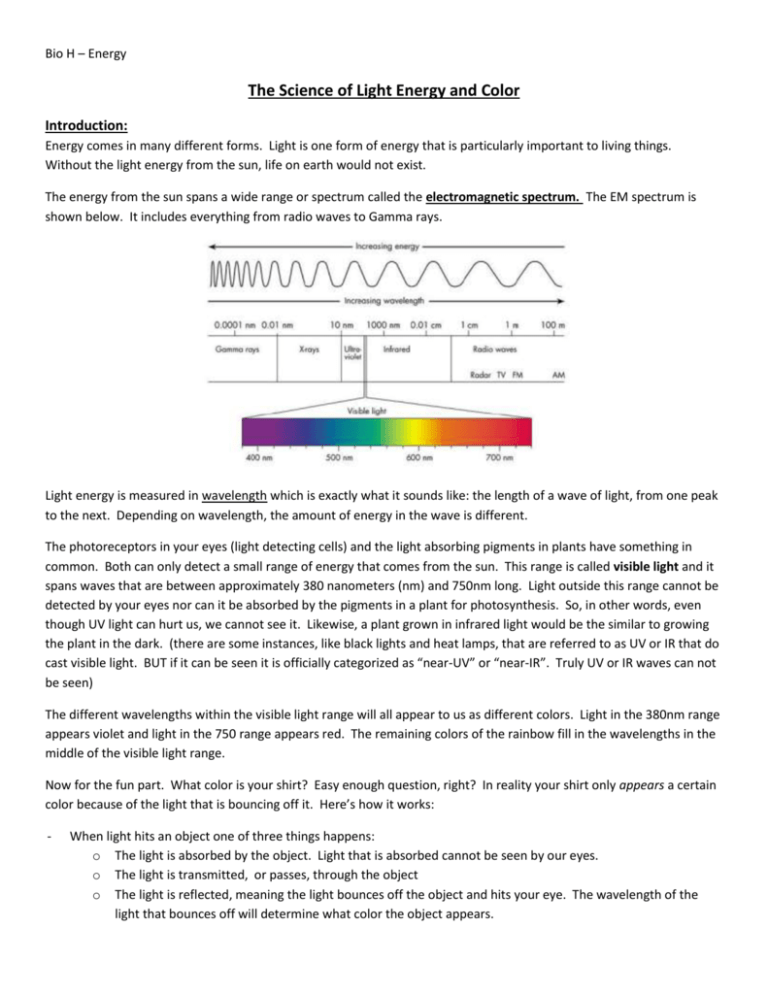
Bio H – Energy The Science of Light Energy and Color Introduction: Energy comes in many different forms. Light is one form of energy that is particularly important to living things. Without the light energy from the sun, life on earth would not exist. The energy from the sun spans a wide range or spectrum called the electromagnetic spectrum. The EM spectrum is shown below. It includes everything from radio waves to Gamma rays. Light energy is measured in wavelength which is exactly what it sounds like: the length of a wave of light, from one peak to the next. Depending on wavelength, the amount of energy in the wave is different. The photoreceptors in your eyes (light detecting cells) and the light absorbing pigments in plants have something in common. Both can only detect a small range of energy that comes from the sun. This range is called visible light and it spans waves that are between approximately 380 nanometers (nm) and 750nm long. Light outside this range cannot be detected by your eyes nor can it be absorbed by the pigments in a plant for photosynthesis. So, in other words, even though UV light can hurt us, we cannot see it. Likewise, a plant grown in infrared light would be the similar to growing the plant in the dark. (there are some instances, like black lights and heat lamps, that are referred to as UV or IR that do cast visible light. BUT if it can be seen it is officially categorized as “near-UV” or “near-IR”. Truly UV or IR waves can not be seen) The different wavelengths within the visible light range will all appear to us as different colors. Light in the 380nm range appears violet and light in the 750 range appears red. The remaining colors of the rainbow fill in the wavelengths in the middle of the visible light range. Now for the fun part. What color is your shirt? Easy enough question, right? In reality your shirt only appears a certain color because of the light that is bouncing off it. Here’s how it works: - When light hits an object one of three things happens: o The light is absorbed by the object. Light that is absorbed cannot be seen by our eyes. o The light is transmitted, or passes, through the object o The light is reflected, meaning the light bounces off the object and hits your eye. The wavelength of the light that bounces off will determine what color the object appears. Bio H – Energy Different wavelengths of light can combine to form different colors. A light that emits (gives off) wavelengths of 400nm (blue) and 600nm (yellow) will look green because the two lights mix together. The sun emits all wavelengths in the visible light spectrum. They all combine together and look white. But REALLY they are many different colors of light together. If the sun’s light shines through a prism or a raindrop, each wavelength is bent at a slight different angle, separating them all out so we can see them individually. This is what makes a rainbow! (a raindrop acts as the prism) In this lab, you will use a device called a spectrometer that will act like a prism and show you all the wavelengths that are REALLY present in several different light sources. So back to that shirt. Since you are sitting in a room with white light, your shirt is being hit with many of the colors of the rainbow. Depending on the material and dyes used to make your shirt, some light might be reflected. It is this reflected light that we actually see. Same goes for a plant. Plants contain several pigment molecules, all of which can absorb certain wavelengths, but each one also reflects certain wavelengths. The reflected light determines the color of the pigment. Finally, the light being emitted can be measured and an intensity graph can be made. An intensity graph shows what wavelengths of light are present AND how intense each is. Intensity of light determines how bright it appears to our eyes. Look at the graph below. Notice we have a peak that ranges from about 525nm to about 600nm. The wavelengths in this range are present. The lack of peaks everywhere else indicates that those wavelengths are not present. Now, imagine if this light ALSO had some light in the 450-460 nm range BUT it wasn’t as bright as the light in the 525 – 600nm range. How would the 450nm-460nm peak differ in both height and width? Answer below. Bio H – Energy Pre lab: Read the entire introduction and examine the figures. Then answer these questions in your journal before beginning your lab. Be sure to answer them in a way that you’ll know what the question was when you look back. Do not just write the question and the answer. 1. What is the relationship between the wavelength of light and the amount of energy it has? 2. Why are biologists particularly interested in the range of light from 380 – 750nm? Provide 2 reasons. 3. Pigments are molecules that absorb light energy. Plants are filled with a pigment called chlorophyll which we know is green. a. What color light is reflected by the chloroplasts? What colors can be absorbed? Which, absorbed light or reflected light, is used to build glucose during photosynthesis? 4. You are walking down the sidewalk in bare feet during a sunny summer day. The light colored concrete is warm, but tolerable. You get to an intersection and step into the black asphalt street. Immediately you start hopping around like a cartoon character as smoke rises from your burning feet. Why was the asphalt so much hotter than the concrete when both were in the sun? 5. What is the difference between wavelength and intensity? a. Discuss which of the above characteristics of light is more important when choosing a light under which a plant will be growing. Procedure: you will be working at stations. Do not proceed to the next station until told to do so. Using the spectroscope: 1. The narrow end of the spectroscope should be placed near your eye while the wide end is aimed at the light. 2. If you look at the wide end of the scope, you will see two slits in the plastic. The short narrow slit should be aimed at the brightest part of the light source. If you are having difficulty, make sure the light source is actually perfectly aligned with this slit otherwise ambient light might be sneaking it. You don’t have to be right on top of the light so if it is too bright and hurting your eyes, take a step back. 3. Once your light source is properly aligned shift the focus of your eyes to the right of the inside of the scope. You should see a “ruler” light you see on your paper and some colored bands. These bands are the actual wavelengths of light coming from the light source. They are aligned on the “ruler” according to the numbers of their actual wavelength. (Ex: you might see a green band in the middle of the 5 and the 6 indicating it is about 550nm) Directions for ALL stations – Use the spectroscopes to figure out what wavelengths are coming from the bulb. Use appropriately colored pencils to put the bands as accurately as possible on the spectrograph (black “ruler”). If the bands are more “smears” then try to make the width of each color and the transitions from one color to the next as accurate as possible. When you have finished with the spectrograph, use it to sketch the intensity graph. Try to accurately place your peaks on the wavelength scale on the X axis and estimate a relative intensity for each band. (ex: if green was brighter than red it should have a higher peak) Bio H – Energy Data: You will cut and paste these graphs into your journal upon completion of this lab Station 1: Compact fluorescent bulb - no special instructions Station 2: LED flashlight – special instructions: hold flashlight about 1ft away from scope Station 3: Black light – special instructions: the intensity is very low for this light. Have someone help you hold the light vertically pressed directly against the slit while you aim the scope toward the window. You’ll need light from the window to see the “ruler” but have to have the bulb pressed against the vertical slit in order to detect the correct light source. Bio H – Energy Station 4: Glow stick - Special instructions: glow sticks have a low intensity. Hold the stick vertically pressed against the light source slit while you aim the scope at the window. (color of glow stick? _______________) Station 5: Sun – Special instructions: DO NOT stare directly at the sun through the scope. Make sure the shades are DOWN and look at sun TROUGH the shades. Obviously, if the sun is not out, you can not look at it. Be patient and see if it comes out from behind a cloud at some point during the period. If, at the end of the period, Mr. Sun has still not cooperated, complete both the spectrograph and intensity graph with predictions of what you expected to see. Station 6: “Daylight” Incandescent bulb – no special instructions Bio H – Energy Discussion Questions: These are the points you should consider when writing the conclusion in your journal. Your conclusion should NOT, however, simply be a list of answers to these questions. 1. Discuss some of the basics of how light works. (Yes, this should echo the introduction but in your own words) 2. Discuss each of the spectra in terms of how beneficial they would be to a typical green plant. 3. Discuss the role pigments play in light absorption in plants and how this effects which types of light are beneficial. 4. Discuss the benefits of a plant having multiple pigments that differ in color instead of just one type.