surface hardening processes
advertisement

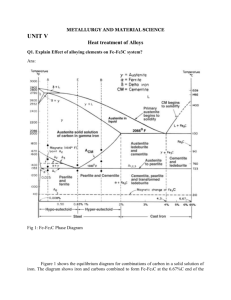
SURFACE HARDENING HEAVY CROSS SECTION - IMPOSSIBLE TO COOL QUICKLY TO PRODUCE A UNIFORMLY MARTENSITIC STRUCTURE THROUGHOUT • A SOFT UNHARDENED CORE DUE TO RELATIVELY SLOW COOLING RATE. CORE WITH FINE PEARLITE, SURFACE MARTENSITICCALLED MASS EFFECT OF HEAT TREATMENT • 0.1% C – TOUGH & SOFT; 0.8% C – HARD & BRITTLE CERTAIN APPLICATIONS WHERE CORE AND SURFACE SHALL HAVE DIFFERENT PROPERTIES. Eg: • Cam, Gears, Shafts to have hard and wear resistant surfaces, but TOUGH, shock resistant cores • HARDNESS- TOUGHNESS- MALLEABILITY- [COMPRESSION ](eg: rolling, forging)- DUCTILITY- [TENSION ](eg: wires drawn) • Carbon or Nitrogen to penetrate to some depth for hardness to increase on surface • Flame or Induction hardening for localised purposes • CASE HARDENING – eg; Wrought Iron to steel by Cementation • Carbon diffused into Iron (of Fe3C structure) CASE HARDENING • Solid, Liquid or Gaseous medium • Release carbon at surface , absorb interstitially to steel- By DIFFUSION • DEPTH DERIVED BY SECOND LAW FICK’S LAWS OF DIFFUSION FIRST LAW dn/dt = no. of moles of B atoms crossing per unit time D= Diffusion coefficient A= Planar area dc/dx= concentration gradient If J = flux flow / unit area per unit time, PACK CARBURISING Carburising Time Packing work in 25 Cr, 20 Ni heat resisting steel boxes with 50 mm gap with carburising material. Heated slowly to 850 – 9250 C, maintained for 8 hrs according to depth needed. Temperature Depth of case • CHARCOAL WITH BARIUM CARBONATE AS ENERGISER (10 to 15%). Process depends on presence of CO 2C + O 2 2 CO At surface, releases C atoms 2CO CO2 + C C dissolved interstitially at surface of steel. Ba CO3 Ba O + CO2 CO2 + C 2CO LIQUID BATH • Mixture of salts of Sodium Cyanide, Sodium carbonate, Sodium/barium Chloride • Melted in pots to 870- 9500 C, work immersed for 5 min to 1 hour • Then basket quenched- hard and clean surface • For shallow- 0.1 to 0.25 ,mm; for small parts GAS CARBURISING • • • • • In batch type or continuous furnaces. Far widely used Clean compact plant Heated to 9000 C for 3 to 4 hours Hydrocarbons methane and propane partly burnt in furnace, diluted with carrier gas to get required carbon POTENTIAL ( ie carbon content maintained in equilibrium in the surface film- of 0.8% desirable) • After carburising, HT necessary to strengthen and toughen the core. • Hardens case too. NITRIDING • Resembles Carburising-(interstitial penetration during heating with nitriding agent) • Hardness depends on the formation of hard nitrides • For alloy steels with Al, Cr, Mo, V (these form strong nitrides) • Plus points: • • • • • No quenching needed, so cracking /distortion least. High surface hardness of 1150 H obtained Resistance to fatigue failure good Resistance to corrosion good, (on unpolished surface) Hardness retained at 5000C ( in carburising falls near 2000C) • Economical for large no. of components • Clean process, (cyaniding with water rinsing environmentally not good) Minus points: • Initial outlay higher than for case hardening • Overheating removes hardness completely. INDUCTION HARDENING AND FLAME HARDENING • USE OF INDUCTION COILS •Gas flame derived •rom acetylene, propane or natural gas . •Manually operated torches used for small areas or localised surfaces. LASER HARDENING • For small, large, surfaces: Locomotive cylinders • Close control of process possible, low distortion, can reach inaccessible regions, • Capital Cost high, but depth of 2.5 mm • Apart from carburizing and Nitriding, • CYANIDING • BORONIZING • CARBONITRIDING HEAT TREATMENT FURNACES • • • • • Batch Continuous Salt bath Fluidized Beds Induction heaters PROTECTION BY METALLIC COATINGS Protection: 1. Direct- by an unbroken film of corrosion-resistant metal covering the article. Eg: tin coatings on steel. 2. Sacrificial- metallic film becomes anodic and dissolves in preference to cathodic surface beneath. Methods: Electroplating, dipping into a bath of molten metal, spraying/volatilizing the protective metal onto the surface, Mechanical Cladding. Laminated Object Manufacturing Profiles of object cross sections are cut from paper or other web material using a laser. The paper is unwound from a feed roll onto the stack and first bonded to the previous layer using a heated roller which melts a plastic coating on the bottom side of the paper. The profiles are then traced by an optics system that is mounted to an X-Y stage. • After cutting of the layer is complete, excess paper is cut away to separate the layer from the web. Waste paper is wound on a take-up roll. The method is selfsupporting for overhangs and undercuts. Areas of cross sections which are to be removed in the final object are heavily cross-hatched with the laser to facilitate removal. It can be time consuming to remove extra material for some geometries • Market segments ranging from concept modeling to very large objects for architectural applications Principle of Ion Plating • MIT Technology Insider publish works on researchprincipally in microstructural design, with emphasis on the microstructure-property relationship in engineered materials. • One area of ongoing research is “grain boundary engineering,” which allows traditional metals and alloys to be dramatically improved in terms of cracking, creep, corrosion, and electro-migration resistance, often by an order of magnitude. These remarkable property enhancements are possible through tailoring the grain boundary network, promoting the development of grain boundaries with “special” crystallography and properties. • Studies on the development of these special boundary networks, the connectivity and percolation behavior of the grain boundary network, and the mechanical properties of these highly engineered materials. • Another area of research is nanocrystalline and amorphous materials. For these unique materials, conventional knowledge of the structure-property relationship breaks down and new physics discovered to explain their unique properties. The traditional as well as modern methods (such as nanoindentation) used to probe the microstructureproperty relationships of these materials. Of particular interest is the transition from a nanocrystalline to an amorphous structure (which occurs at grain sizes near ~2 nm) and the manner in which properties change through this regime. • scanning electron microscopy and Energy Dispersive X-ray Microanalysis (EDX). • Eg: 13A plug- List of Parts 1- Fuse Holder 2- Cover Screw 3- Earth Pin 4- Wire fixing screw 5- Live and Neutral Pins 6- Fuse holder rivet CLADDING • Mainly in the manufacture of clad sheets- employed prior to the final manufacturing stages of a component • Base metal sandwiched between the pieces of the coating metal, sandwich then rolled to the required thickness • In some, film of protective metal sprayed onto the base surface & then rolled. • ALCLAD- Duralumin coated with aluminium (0.01 mm thick) • MS clad with SS • But, damage to protective skin during fabrication CERAMICS earthenware, stoneware, porcelain • , Frederick Carlton Ball (1911-1992) was a prize fighter who went by the name "California Gene Tunney". • Teacher at California College of Arts and Crafts, Southern Illinois University, the University of Puget Sound, Mills College, Oakland and the University of Southern California, • Wrote book: 'Decorating Pottery with Clay, Slip and Glaze'