Technology for Long Last Senses
advertisement
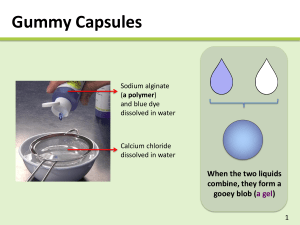
Technology for Long Last Senses Assoc. Prof. Ubonthip Nimmannit, Ph.D. April 2nd, 2010 National Nanotechnology Center NSTDA Pathumthani, Thailand Fragrances • Topnote • Middle note • Lasting note (Base note) Topnote • First impression • High volatility, coefficients ranging 1-14 • No residual scent after 2 h – Citrus, fruity, green note – Ginger, galanga, cardamom Middle note • Establishing the whole fragrance • Medium volatility, coefficients ranging 15-60 • Last for 2-6 h – Jasmine, rose, aldehyde, spicy note – Tuberose, coriander Lasting note (Basic note or Fixer) • Low volatile fixatives, coefficients ranging 61-100 • Last for more than 6 h – Oakmoss, woody note, animal note, civet absolute, myrrh, velvety balsamic scent Basic note/ Fixation – Musk ambrette – Ambergris extract – Ethyl vanillin/ vanillin – Methyl nonyl acetaldehyde – Fixateur 404 Persistence of Perfumes • Coefficient: ↑ nature of evaporation ↓ value of the threshold concentration Volatility • Odor intensity • Threshold value • Odor tonality Stability of Fragrance • Change of odor • Change of color Stability factors • • • • • Oxygen Light Temperature Humidity pH of product Microcapsules • Small particles (1-100 μm) • Active agent surrounded by polymeric membrane • Protect from oxidation (by heat, light, humidity, exposure to other substances) • Prevent evaporation • Control release rate Microcapsules • Released by – Mechanical – Temperature – Diffusion – pH – Biodegradation – Dissolution Techniques of encapsulation (Micro/ Nano) • Phase separation techniques • Interfacial polymerization techniques • Mechanical techniques Phase separation techniques • Aqueous phase separation – Simple coacervation – Complex coacervation • Nonaqueous phase separation – Non solvent addition – Temperature reduction Complex coacervation Positive charge polymer-negative charge polymer Phase separation Colloidal rich phase + equilibrium liquid stirred Microcapsules Nanocapsules dry Interfacial Polymerization Technique Monomer A + Monomer B (Hydrophobic liquid) (Hydrophilic liquid) Emulsifying Polymer AB Stirring Microcapsules + by product Mechanical Techniques • Spray dry • Atomization • etc. Selection of the technique and shell material – – – – – – Application of products Physical and chemical stability Concentration Desire particle size Release mechanism Manufacturing cost Oil phase (HMDI) + Aqueous phase 1 (PVA) Formation of oil/water emulsion Aqueous phase 2 (PEG 400, DBTDL) Urethane formation Aqueous phase 3 (EDA) Polymerization and shell formation Urea formation Aqueous phase 4 (HYD) Urea formation Separation/Washing Microencapsulation process of limonene by interfacial polymerization. Rodrigues, et al., Ind. Eng. Chem. Res. 2008, 47, 4142–4147 Optical microscopy of microcapsules solution. Magnification: (a) 20x (b) 100x. Textile Impregnated with Microcapsules Characterization. SEM Analysis Rodrigues, et al., Ind. Eng. Chem. Res. 2008, 47, 4142–4147 Macrocapsules • Diameter over 1000 mm Polysulfone capsules containing different vanillin concentrations. show a different porosity distribution, T. Gumํ et al. / Desalination 245 (2009) 769–775 Polymeric microspheres • Fragrance incorporated in the polymer – Controlled by • Initial loading of fragrance • Ability of fragrance to diffuse through polymeric barrier – Driving force • Interaction between fragrance molecule and polymer matrix • Vapor pressure of fragrance SEMandTEMimages of spheres obtained with the starting polymer concentrations of (a) 2000–16,000 ppm, (b) 18,000ppmand (c) 24,000– 28,000 ppm. (a) SEM, (b) TEM and (c) AFM images of menthol-encapsulated polymeric nanoparticle suspension (polymer blend : EC, HPMC, PV(OH) A. Sansukcharearnpon et al. / Int J Pharm xxx (2010) xxx–xxx Multiarm star-block copolymer • Multiarm star-block copolymer compared to dendrimer – Less complex synthesis – Less time consuming – Lower cost Limitation – Lower loading capacity than dendrimer Multiarm star-block copolymer Hydrophobic arms Hydrophilic arms Hyperbranched core structure Amphiphilic multiarm star-block copolymers with a hydrophilic inner and hydrophobic outer shell (top) and with a hydrophobic inner and hydrophilic outer shell (bottom). Ternat et al., Macromolecules, Vol. 41, No. 19, 2008 Multiarm star-block copolymer Average structures of the Boltorn H40 core and amphiphilic multiarm star-block copolymers H40-(PnBuMA)p-b-(PPEGMA)q and H40-(PCL)pb-(PAA)q. Ternat et al., Macromolecules, Vol. 41, No. 19, 2008 Multiarm star-block copolymer Benzyl acetate/ H40-(PCL)24-b-(PAA)82 Benzyl acetate Comparison of the evaporation rates of benzyl acetate from an aqueous solution (containing 5% of ethanol) in the presence and absence of amphiphilic multiarm star-block copolymer H40-(PCL)24-b-(PAA)82. • • • • Alginate complex capsules containing eucalyptus oil Interfacial insolubilization reaction Release by crashing the capsule between fingers Closed capsule wall Optimum condition – Concentration of alginate – Concentration of calcium salt – Cross-linking time The capsules were prepared at concentrations of 1.5% sodium alginate and 1.0% calcium chloride, and the cross-linking time of 20 min. Microphotographs of alginate complex capsules before (a) and after (b) the hardening process (/80). C.P. Chang, T. Dobashi / Colloids and Surfaces B: Biointerfaces 32 (2003) 257/262 Time courses of oil release from capsules at incubation process for the samples prepared at various conditions: At different concentrations of sodium alginate at constant calcium chloride concentration of 1 w/v% at cross-linking time of 20 min. The symbols (circle), (triangle), (square) and (diamond) denote concentrations of sodium alginate of 0.25, 0.50, 1.0 and 2.0%, respectively. Light induced controlled release of fragrances Alkyl phenyl ketones serve as delivery systems for fragrance molecules upon exposure to natural sunlight Preparation of alginate nanocapsules containing turmeric oil Sodium alginate crosslink with calcium chloride alginate nanocapsule Lertsutthiwong P., Noomun K., Jongaroonngamsang N., Rojsitthisak P., Nimmannit U. 2008. Preparation of alginate nanocapsules containing turmeric oil. Carbohydrate Polymers. 74. 209–214. Morphology and size of turmeric oil-loaded alginate nanocapsules TEM characterization of nanocapsules, indicating an average size of about 95 nm. Thank you