Bonding: General Concept
advertisement

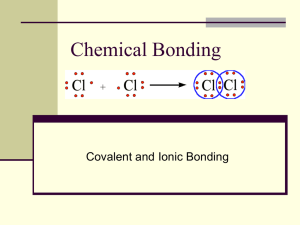
Bonding: General Concept • • • • • • • • • • • Types of Chemical Bonds The Formation of Ions and Their Electron Configurations Ionic Size and Charges, and the Relative Strength of Ionic Bonds Energy of Formation of Ionic Compounds – The Born-Haber Cycle Covalent Bond: Electronegativity, and Bond Polarity Lewis Structures and the Octet Rule Exceptions to the Octet Rule Resonance Lewis Structures Bond Energies The Calculation of Enthalpy of Reaction from Bond Energy The VSEPR Model and Molecular Shapes Ionic bonds • electrostatic attractions between cations and anions; • Bonds formed between metals and nonmetals • Reactions that produce ionic bonds involve the transfer of one, two, or three electrons from a metal atom to nonmetal atom Covalent Bonds • One, two or three pairs of electrons shared between two atoms • Bonds between two nonmetals or between semimetal and nonmetal atoms • Bonds are formed when two atoms share electron pairs The Formation of Ions • Ions are formed when metals react with nonmetals, in which the metal atoms donate their valence electrons to the nonmetals; • Atoms of the representative metals lose their valence electrons to become cations that have the electron configurations of noble gases; • The nonmetal atoms gain a number of electrons to become anions that also have the electron configuration of the noble gases; Formation of Cations • From the alkali metals (1A): – M M+ + e• From the alkaline Earth metals (2A): – M M2 + + 2e• From Group 3A metals: M M3+ + 3e- ; Formation of Anions • From the halogen family (VIIA): – X + e- X • From the oxygen family (VIA): – X + 2e- X2• From N and P (in Group VA): – X + 3e- X3- Common Ions of the Representative Elements • Ions with the electron configuration of He = 1s2 – Li+ and H• Ions with the electron configuration of Ne = 1s2 2s2 2p6 – Na+, Mg2+, Al3+, F-, O2-, and N3• Ions with the electron configuration of Ar = 1s2 2s2 2p6 3s2 3p6 – K+, Ca2+, Sc3+, Cl-, S2-, and P3• Ions with the electron configuration of Kr = 1s22s22p63s23p64s23d104p6 – Rb+, Sr2+, Y3+, Br-, and Se2-; • Ions with the electron configuration of Xe = 1s22s22p63s23p64s23d104p65s24d105p6 – Cs+, Ba2+, La3+, I-, and Te2-; Cations From the Transition Metals • Transition metal atom loses variable number of electrons • Cations derived from transition metals have variable charges • Cations of transition metals do not acquire the electron configurations of noble gases • Examples: – Cr Cr2+ + 2e-; Cr2+: [Ar] 3d4 – Cr Cr3+ + 3e-; Cr3+: [Ar] 3d3 – Fe Fe2+ + 2e-; Fe2+: [Ar] 3d6 – Fe Fe3+ + 3e-; Fe3+: [Ar] 3d5 • (Note that these cations do not have the 4s electrons in their electron configurations.) Charge Density and The Strength of Ionic Bonds Relative sizes of isoelectronic ions: • • Al3+ < Mg2+ < Na+ < Ne < F- < O2- < N3-; Sc3+ < Ca2+ < K+ < Ar < Cl- < S2- < P3-; Trend of ionic radii within a group: • • Li+ < Na+ < K+ < Rb+ < Cs+; F- < Cl- < Br- < I-; Ionic Bond Strength: • • Strength of Ionic bonds is related to charge density of the ions; Greater charge and smaller ions lead to stronger ionic bond; Ionic Bond Strength and Lattice Energy • The strength of ionic bonds is associated with the magnitude of the lattice energy • Lattice energy - energy released when gaseous ions combine to form a mole of solid ionic compound: – M+(g) + X-(g) MX(s); UL = lattice energy • Example: Na+(g) + Cl-(g) NaCl(s); • Li+(g) + F- (g) LiF(s); UL = -787 kJ/mol UL = -1047 kJ/mol • Lattice energy = k(q1q2/r); – where q1 and q2 are charge magnitude on ions, r is the internuclear distance, and k is the proportionality constant. – Lattice energy increases with charge magnitude but decreases with ionic size Lattice Energies of Some Ionic Compounds • Lattice Energy, UL(kJ/mol) – The energy required to separate a mole of ionic solids into the gaseous/vapor ions; – MX(s) M+(g) + X-(g) • • • • • • Mn+/XnLi+ Na+ K+ Mg2+ Ca2+ F1047 923 821 2957 2628 Cl853 787 715 2526 2247 Br807 747 682 2440 2089 I757 704 649 2327 2059 O22942 2608 2311 3919 3570 ______________________________________________________________________ The Born-Haber Cycle for NaCl • Na+(g) + Cl(g) _______________ • -349 kJ + • +496 kJ _______ Na (g) + Cl (g) • Na(g) + Cl(g)___________ • +121 kJ • Na(g) + ½Cl2(g)________ ? kJ • +108 kJ • Na(s) + ½Cl2(g)________ • -411 kJ • NaCl(s)_________________ Chemical Processes in the Formation of NaCl • Na(s) Na(g); • ½Cl2(g) Cl(g); • Na(g) Na+(g) + e-; • Cl(g) + e- Cl-(g); • Na+(g) + Cl-(g) NaCl(s); • Na(s) + ½Cl2(g) NaCl(s); DHs = +108 kJ ½BE = +121 kJ IE = +496 kJ EA = -349 kJ UL = ? kJ DHf = -411 kJ – UL = DHf – (DHs + ½BE + IE + EA) (DHs = Enthalpy of sublimation; IE = Ionization energy; BE = Bond energy; EA = Electtron affinity; UL = Lattice energy; DHf = Enthalpy of formation) The Born-Haber Cycle for LiF • Li+(g) + F(g) _______________ • -328 kJ + • +520 kJ _______Li (g) + F (g) • Li(g) + F(g)___________ • +77 kJ • Li(g) + ½F2(g)________ ? kJ • +161 kJ • Li(s) + ½F2(g)________ • -617 kJ • LiF(s)_________________ Chemical Processes in the Formation of LiF • Li(s) Li(g); • ½F2(g) F(g); • Li(g) Li+(g) + e-; • F(g) + e- F-(g); • Li+(g) + F-(g) LiF(s); • Li(s) + ½F2(g) LiF(s); DHs = +161 kJ ½BE = +77 kJ IE = +520 kJ EA = -328 kJ UL = ? DHf = -617 kJ – UL = DHf – (DHs + ½BE + IE + EA) (DHs = Enthalpy of sublimation; IE = Ionization energy; BE = Bond energy; EA = Electtron affinity; UL = Lattice energy; DHf = Enthalpy of formation) The Born-Haber Cycle for MgO • Mg2+(g) + O2-(g) _____________ • +737 kJ • Mg2+(g) + O(g)________ • +2180 kJ • Mg(g) + O(g)_________ • +247 kJ • Mg(g) + ½O2(g)________ • ? kJ +150 kJ • Mg(s) + ½O2(g)________ • -602 kJ • MgO(s)_________________ Chemical Processes in the Formation of MgO • Mg(s) Mg(g); DHs = +150 kJ • ½O2(g) O(g); ½BE = +247 kJ • Mg(g) Mg2+(g) + 2e-; IE = +2180 kJ • O(g) + 2e- O2-(g); EA = +737 kJ • Mg2+(g) + O2-(g) MgO(s); UL = ? kJ • Mg(s) + ½O2(g) MgO(s); DHf = -602 kJ – UL = DHf – (DHs + ½BE + IE + EA) (DHs = Enthalpy of sublimation; IE = Ionization energy; BE = Bond energy; EA = Electtron affinity; UL = Lattice energy; DHf = Enthalpy of formation) Covalent Bonds • Covalent bonds – A bond between two nonmetal atoms or between a semimetal and a nonmetal atoms; – Bonded atoms may share one, two, or three pairs of electrons. • Nonpolar covalent bonds are formed between identical atoms or if bonded atoms have the same electronegativity. • Polar covalent bonds are formed when bonded atoms have different electronegativity; • Polar covalent bonds are covalent bonds with partial ionic character Potential Energy Diagram for Covalent Bond Formation Potential energy of H-atoms during the formation of H2 molecule Lewis Model for the Formation of Covalent Bonds and Covalent Molecules General trends: • Electronegativity increases from left to right along a period • For the representative elements (s and p block) the electronegativity decreases as you go down a group • The transition metal group is not as predictable as far as electronegativity. Electronegativity • Electronegativity is the relative ability of a bonded atom to attract shared electrons closer to itself. – Electronegativity increases going across a period and decreases going down a group. – Most electronegative elements – at top right corner of PT – Least electronegative elements – at bottom left corner of PT – Fluorine (F) is most electronegative with EN value of 4.0 – Francium (Fr) is least electronegative with a value of 0.7 • The polarity of a covalent bond depends on the electronegativity difference (DEN) of the two bonded atoms. Electronegativity and Bond Polarity Compound Electronegativity Difference Type of Bond F2 HF LiF 4.0 - 4.0 = 0 4.0 - 2.1 = 1.9 4.0 - 1.0 = 3.0 Nonpolar covalent Polar covalent Ionic (noncovalent) •In F2 the electrons are shared equally between the atoms, the bond is nonpolar covalent •In HF the fluorine atom has greater electronegativity than the hydrogen atom. •The sharing of electrons in HF is unequal: the fluorine atom attracts electron density away from the hydrogen (the bond is thus a polar covalent bond) Electronegativity and bond polarity The H-F bond can thus be represented as: •The 'd+' and 'd-' symbols indicate partial positive and negative charges. •The arrow indicates the "pull" of electrons off the hydrogen and towards the more electronegative atom. •In lithium fluoride the much greater relative electronegativity of the fluorine atom completely strips the electron from the lithium and the result is an ionic bond (no sharing of the electron) Predicting Bond Type From Electronegativity A general rule of thumb for predicting the type of bond based upon electronegativity differences (DEN): • If DEN between the two atoms is 0-0.5, the bond is non-polar covalent; • If DEN between the two atoms is greater than 0.5, but less than 1.5, the bond is polar covalent • If DEN between the two atoms is 1.5, or greater, the bond is ionic Bond Length The bond length is defined as the distance between the nuclei of the two atoms involved in the bond. In general, the larger the atoms involved in a bond, the longer the bond length, and the more bonds between two atoms, the shorter the bond length. . Bond Energy Bond energy is the energy required to break the bond(s) between two atoms. In general, the shorter the bond, the higher the bond energy. Lewis Symbols for Atoms and The Formation of Covalent Molecules Lewis Symbols for O, F, and Na How to draw a Lewis structure of a simple molecular compound or polyatomic ion? A Lewis structure can be drawn for a molecule or ion by following three steps: 1. Calculate the number of valence electrons (including charges, if any) 2. Write skeleton structure – Lowest EN (electronegative) atom, largest atom, and/or atom forming most bonds is usually the central atom – Hydrogen and Fluorine cannot be the central atom. – Connect all atoms with a single bond – using a line or two dots. – In oxoacids, such as sulfuric acid (H2SO4), the oxygen bonds to central atom and the H to oxygen. – Compounds are usually compact and symmetrical structures 3. Count how many electrons have been used – Distribute remaining electrons to terminal atoms filling them until each has eight electrons (octet rule), unless it is hydrogen atom. 4. Central atom octet is filled last – Any remaining electrons become lone pairs on central atom. – If central atoms do not have an octet, move from terminal atoms one pair at a time to form double and triple bonds. Covalent Bonds and Lewis Structures Some Molecules Lewis Structures of HF, H2O, NH3, & CH4 Lewis Structures of CH4, NH3 and H2O Lewis Structures of CO2, HCN, and C2H2 Lewis Structures of Other Covalent Molecules Assigning Formal Charges in Lewis Dot Structures? • Formal Charge – To determine the formal charge of an atom from a Lewis dot structure we need to assign each electron to an atom in the structure. To do this we use the following rules: 1. All nonbonding electrons (unshared electrons) are assigned to the atom on which they are found. 2. Each atom in a bond is assigned ½ of the total number of electrons in the bond (i.e. for a single bond each atom is assigned 1 electron, for a double bond each atom is assigned 2 electrons, etc.) 3. For each atom the number of electrons assigned in the above steps is subtracted from the number of valence electrons in the atom. Choosing the correct or best Lewis structures based on formal charges • If two or more Lewis dot structures can be drawn which satisfy the octet rule, the most stable one will be the structure where: 1. 2. The formal charges are as small as possible. Any negative charges are located on the more electronegative atoms. Assigning Formal Charges Which Lewis structures of CO2 & N2O are correct? Resonance Lewis Dot Structures for CO32- Resonance Lewis Structures of PO43- Assigning Appropriate Formal Charges Lewis Structures, Molecular Shapes & Polarity The Shapes of Methane and Ammonia Molecules The Shape of Water Molecules Structures and Shapes of Formaldehyde and Ethylene Bond Length and Bond Energies • Bond length (pm) and bond energy (kJ/mol) • Bond Length Energy Bond Length Energy _________________________________________________________________________________________________________ • • • • • • • • • • • • • • • • H─H C─C N─N O─O F─F Cl─Cl Br─Br I─I C─C C─N C─O O─O O=O C─Br C─I 74 154 145 148 142 199 228 267 154 147 143 148 121 194 214 436 348 170 145 158 243 193 151 348 308 360 145 498 288 216 H─C H─N H─O H─F H─Cl H─Br H─I 109 101 96 92 127 141 161 413 391 366 568 432 366 298 C─C C=C C≡C C─S C─F C─Cl N─N N≡N 154 134 120 182 135 177 145 110 348 614 839 272 488 330 170 945 Bond Breaking and Bond Formation in the Reaction to form H2O Using Bond Energies to Calculate Enthalpy of Reactions in Gaseous State • Chemical reactions in the gaseous state only involve: – the breaking of covalent bonds in reactants and – the formation of covalent bonds in products. • Bond breaking requires energy input - an endothermic process), • while bond formation releases energy – an exothermic process; DHreaction = S(Energy of bond breaking) + S(Energy of bond formation) Calculation of Reaction Enthalpy Using Bond Energies • Use bond energies to estimate the DH for the reaction: CH3OH(g) + 2 O2(g) CO2(g) + 2H2O(g); • • • • S(energy of bond breaking) (in kJ) = 3 x BE(C─H) + BE(C─O) + BE(O─H) + 2 x BE(O═O) = (3 x 413) + 358 + 467 + (2 x 495) = 3054 kJ S(energy of bond breaking) (in kJ) = 2 x -BE(C═O)* + 4 x -BE(O─H) = (2 x -799) + (4 x -495) = -3578 kJ DHreaction = 3054 + (-3578) = -524 kJ Molecular Shapes of BeI2, HCl, IF2-, ClF3, and NO3- The VSEPR Shapes Linear and Trigonal Planar Electron-Pair Geometry The Tetrahedral Electron-Pair Geometry Trigonal Bipyramidal Electron-Pair Geometry The Octahedral Electron-Pairs Geometry