6_Ugochukwu Aronu
advertisement

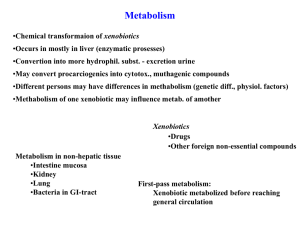
1 Solvent Development for CO2 Capture Ugochukwu E. Aronu and Hallvard F. Svendsen Norwegian University of Science and Technology (NTNU) Trondheim, NORWAY J, 2008 U 2 Outline • Research Plan Overview • CO2 Capture Amine Solvent Review • Structure – pKa Relationship • VLE Experiment • Summary 3 Research Plan Overview Review of CO2 capture solvents -Properties of an ideal solvent -Suggested/Future amine solvents Screening Tests - Amine systems will be screened for absorption capacity and absorption rate VLE Experiments -VLE experiment on selected system -Thermodynamic model of VLE results Kinetics Experiments - Rate determination - Kinetics model Physicochemical Properties Determination -pKa, Solubility, Viscousity, Activity Coefficient, Density Simulations-HSYS 4 Objective Objective of this project is to develop solvent(s) that will be able to meet considerably the present and/or future requirements of an ideal solvent for carbon dioxde capture. Some of the key requirements of the solvent(s) include: High absorption capacity High absorption rate Low regeneration energy Chemically stable Non-corrosive Cheap Environmentally friendly 5 CO2 Capture Solvents Review • Ideal Solvent Properties of Interest Here we look at the desired properties of an ideal solvent and how such property is related to CO2 capture process - Dissociation Constant (pKa) - Solubility - Degradation - Foaming - Reaction Kinetics - Toxicity/Environmental properties • Solvent Collection – Commercial Solvent – Single Solvent – Blended Solvent - Heat of reaction - Molecular weight - Corrosion - Vapour Pressure - Cost 6 Commercial Amine Processes & Solvents Amine Process/Solvent Developer Solvent Composition Cansolv® CO2 capture System (Absorbent DC 101TM) Cansolv Technologies Inc. Tertiary amine and promoter mixture Flour Daniel Econamine FGSM process Dow Chemical and Union Carbide 30wt% MEA with inhibitor Kansai-Mitsubishi proprietary Carbon Dioxide Recovery Process (KM-CDR) KS-1 Mitsubishi Heavy Industries (MHI) Ltd and Kansai Electric Power Co., Ltd Hindered amine and promoter mixture BASF activated methyldiethanolamine (aMDEA) BASF MDEA mixture with accelerator, Piperazine Kerr-McGee/ABB Lummus Crest Technology Kerr-McGee 15-20wt% MEA without inhibitors UCARSOLTM AP 800 Series Dow Chemical Company Accelerated MDEA (MDEA/Piperazine mixture) ADIP-X Technology Shell Global Solution Accelerated MDEA (MDEA/Piperazine mixture) Just CatchTM Aker Kvaerner 7 Some Single Amine Solvents Name Monoethanolamine 2-Ethylaminoethanol Monomethylethanolamine Methyldiethanolamine 2-Butylaminoethanol N-Aminoethylethanolamine Piperazine Ammonia Methylamine Ethylamine Dimethylamine Trimethylamine Piperidine Morpholine Pyrrolidine 2,2,6,6-Tetramethyl-4-piperidinol 1-amino-2-propanol 2-amino-2-methylpropanol Diethanolamine Diisopropanolamine N-n- butylethanolamine Triethanolamine 1,3-propanediamine 2-(2-aminoethoxy)ethanol 2-(diethylamino)ethanol 2-(diisopropylamino)ethanol 2-(tert-butylamino)ethanol Ethylenediamine 2-Amino-2-Methyl-1,3-Propanediol CAS NO 141435 110736 109831 105599 111751 111411 110850 7664417 74895 75047 124403 75503 110894 110918 123751 2403885 78966 124685 111422 110974 36386739 102716 109762 929066 100378 96800 4620706 107153 115695 Name CAS NO 2-Amino-2-Ethyl-1,3-Propanediol N-(2-aminoethyl)-1,3-propanediamine 1,8-p -menthanediamine 2-piperidineethanol 2-amino-2-methylpropionic acid 2-amino-2-methyl-1-propanol 1-amino-1-cyclopentanecarboxylic acid 1-amino-1-cyclohexanecarboxylic acid 2-amino-2-phenylpropionic acid Pipecolinic acid 2-amino-2-hdroxymethyl-1,3-propanediol Tetraethylenepentamine Bis(3-(dimethylamino)propyl)amine Quinuclidine 2-Piperidinemethanol 2-Ethoxyethanol tertiarybutylaminoethanol Diethylmonoethanolamine 2-methyl-3-hydroxy piperidine 3-amino-3-methyl-1-butanol 2-amino-2-methyl-1-butanol 3-amino-3-methyl-2-pentanol 1-methyl-3-hydroxypiperidine dimethylethanolamine 3-amino-1,2-propanediol Diethylenetriamine Triethylenetetramine Tetraethylenepentamine hexamethylenediamine 115708 13531527 80524 1484840 62577 124685 52528 21121379 565071 535751 77861 112572 6711484 100765 3433372 110805 4620706 100378 916083288 42514501 10196302 164656820 3554743 108010 616308 111400 112243 112572 124094 8 Structure - pKa Relationship Chain Length Effect CAS Registry No. Molecular Formula Monoethanolamine (MEA) 141-43-5 C2H7NO 3-amino-1-propanol (MPA) 156-87-6 C3H9NO 4-amino-1-butanol 13325-10-5 C4H11NO 5-amino-1-pentanol 2508-29-4 C5H13NO Ethylenediamine (EDA) 107-15-3 C2H8N2 1,3-propanediamine 109-76-2 C3H10N2 1,4- diaminobutane 110-60-1 C4H12N2 1,5-Pentanediamine 462-94-2 C5H14N2 hexamethylenediamine (HMDA) 124-09-4 C6H16N2 Name Structural Formula Type pKa** pKa* 25 C Calculated 25oC o Primary Amines Primary Alkanolamines NH2 OH NH2 OH NH2 OH NH2 OH p 9.5 9.16±0.10 p 9.96 9.91±0.10 p 10.38 @ 20oC 10.32±0.10 p 10.46 @ 23oC 10.47±0.10 p(2) pK1 = 9.92 pK2 = 6.86 9.89±0.10 p(2) pK1 =10.55 10.43±0.10 pK2 = 8.88 p(2) pK1 = 10.8 pK2 = 9.63 p(2) pK1 = 10.05 10.85±0.10 pK2 = 10.93 p(2) pK1 = 11.02 10.92±0.10 pK2 = 10.24 Primary Diamines H2N NH2 NH2 NH2 NH2 NH2 NH2 NH2 NH2 NH2 10.68±0.10 9 Structure - pKa relationship Multifunctional Amine Group Effect Name 1,4-Butanediamine CAS Registry No. Molecular Formula 110-60-1 C4H12N2 Structural Formula Type NH2 p(2) pK1 = 10.8 pK2 = 9.63 10.68±0.10 p(2), s pK1 = 9.94 pK2 = 9.13 pK3 = 4.34 10.16±0.10 NH2 Diethylenetriamine(DETA) 111-40-0 C4H13N3 NH NH2 1,5-Pentanediamine N-(2-aminoethyl)-1,3propanediamine (AEPDA) 462-94-2 C5H14N2 13531-52-7 C5H15N3 Hexamethylenediamine (HMDA) 124-09-4 C6H16N2 N-(3-aminopropyl)-1,3propanediamine 56-18-8 C6H17N3 Triethylenetetramine (TETA) NH2 NH2 NH2 C6H18N4 pK1 = 10.05 10.85±0.10 pK2 = 10.93 p(2), s NH NH2 NH NH2 p(2) pK1 = 11.02 10.92±0.10 pK2 = 10.24 p(2), s pK1 = 10.65 pK2 = 9.57 10.71±0.10 pK3 = 7.72 NH2 NH NH 10.17±0.10 NH2 NH2 NH2 112-24-3 p(2) NH2 pKa** pKa* 25 C Calculated 25oC o NH2 p(2), s(2) pK1 = 9.92 pK2 = 9.20 pK3 = 6.67 pK4 = 3.32 10.05±0.19 10 Structure - pKa relationship Alkyl Group Effect CAS Registry No. Molecular Formula Trimethylamine 75-50-3 C3H9N Triethylamine 121-44-8 C6H15N Name Structural Formula Type pKa** Calculated pKa* 25 C 25oC o Tertiary amine N t 9.80 9.75±0.28 t 10.75 10.62±0.25 t 10.66 9.99±0.50 t 9.23 8.88±0.28 t 9.75 9.79±0.25 N Tripropylamine 102-69-2 C9H21N N Tertiary alkanolamine dimethylethanolamine (DMEA) 108-01-0 C4H11NO N OH 2-(diethylamino)ethanol (DEAE) 100-37-8 C6H15NO N OH 3-(diethylamino)-1-propanol 622-93-5 C7 H17NO N 1-(diethylamino)-2-propanol 4402-32-8 t 10.13±0.25 t 10.27±0.25 OH C7H17NO N OH 11 Structure - pKa Relationship Hydroxyl Group Effect-Primary Alkanolamine CAS Registry No. Molecular Formula 2-amino-2-methylpropanol (AMP) 124-68-5 C4H11NO 2-amino-1-butanol 96-20-8 Name Structural Formula Type pKa** Calculated pKa* 25 C 25oC o Primary Alkanolamines NH2 p,(SH) 9.72 9.20±0.25 OH OH C4H11NO p 9.67 @ 20oC 9.27±0.10 p 9.62 @ 20oC 9.18±0.10 NH2 Monoisopropanolamine (MIPA) 2-Amino-2-Methyl-1,3Propanediol (AMPD) OH 78-96-6 C3H9NO H2N NH2 115-69-5 C4H11NO2 HO OH p,(SH) 8.80 8.90±0.29 p,(SH) 8.80 8.86±0.29 p,(SH) 8.07 7.78±0.29 HO 2-Amino-2-Ethyl-1,3Propanediol (AEPD) 115-70-8 C5H13NO2 HO NH2 NH2 2-amino-2-hdroxymethyl-1,3propanediol (AHPD) 77-86-1 C4H11NO3 HO OH HO 12 VG14 Safety Valve VLE Apparatus VP1 VP2 VG17 TI N2 or CO2 VG7 P T Gas Pump 2 VG15 VG3 GS3 VG1 GS2 GS1 VG2 VG6 VG16 VG4 VG5 VG13 P T Cooler VG8 Gas Pump 1 FT VG9 CO2 Analyzer Equilibrium Cell LS2 CO2 LS1 Hygromete r Gear Pump FI VG12 Oil Bath Amine solution VG10 MF C 2 P T VG11 MF C 1 N2 TI VL2 VL1 Feed Pump Cooler 13 VLE Test Result VLE 30wt% MEA 40oC 100000.0 10000.0 PCO2(kPa) 1000.0 Loadingfresh 100.0 Jou, et. al (1995) Loadingtitration 10.0 Sholeh 1.0 Shen and Li (1992) 0.1 0.0 0.0 0.0 0.2 0.4 0.6 Loading(molCO2/molMEA) 0.8 1.0 14 Summary • Several amines solvents have been suggested for CO2 Capture • It appears there is relationship between amines structures and their pKa value • Effort is to explain this relationship using electron donation and withdrawal through bonds and solvation effect • To know the benefit that could be derived from this relationship in CO2 capture process, particularly in solvent selection • VLE Experiments continues 15 Thank You