Phase 1
advertisement
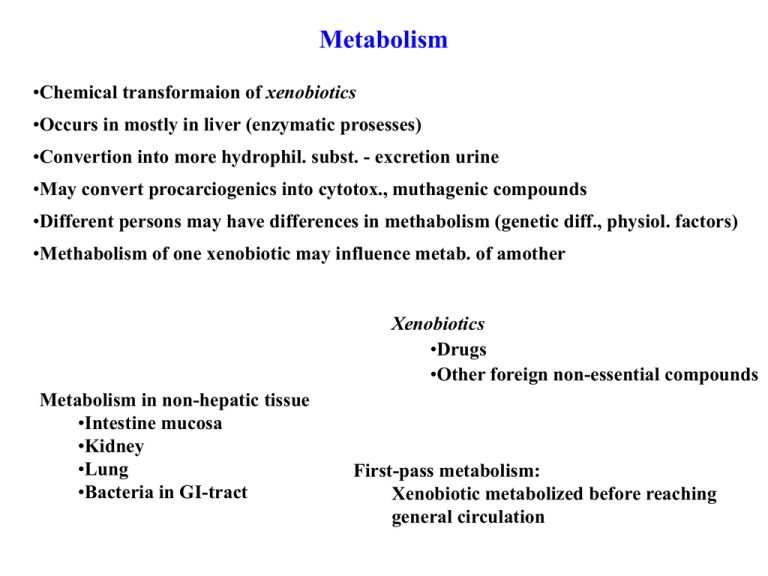
Metabolism •Chemical transformaion of xenobiotics •Occurs in mostly in liver (enzymatic prosesses) •Convertion into more hydrophil. subst. - excretion urine •May convert procarciogenics into cytotox., muthagenic compounds •Different persons may have differences in methabolism (genetic diff., physiol. factors) •Methabolism of one xenobiotic may influence metab. of amother Xenobiotics •Drugs •Other foreign non-essential compounds Metabolism in non-hepatic tissue •Intestine mucosa •Kidney •Lung •Bacteria in GI-tract First-pass metabolism: Xenobiotic metabolized before reaching general circulation First-pass metabolism: Xenobiotic metabolized before reaching general circulation A) Metab. lungs (inhaled subst) Intestine mucosa, GI bacteria B) G e n e ra l C irc u la tio n GI L iv e r A b s o rb u n d e r tu n g A b s o rb fro m re c tu m Pathways of metabolism Phase 1: Biotransformation Attachment of new functional groups, transformation of exist. funct. groups oxidation, reduction, hydroxylation, hydrolysis etc. Phase 2: Conjugation. Masking of an exist. funct. group by for instance acetylation, glycosylation, attachment amino acid etc More hydrophilic drug Renal excretion N CH3 NH NH C o n ju g a tio n g lu cu ro n ic a cid D e m eth y la tio n HO O M o rp h in e OH P h a se 1 HO O OH P h a se 2 HO O OH HO O O OH C O 2H Phase 1 Metabolism by cytochrome P450 enzyme systhem (CYP450) •Located in endoplasmatic reticulum (liver and other cells) •Electron transport systhem - oxidation, monooxygenase •Heme protein + flavoprotein •Capablee of oxidation - many differen xenobiotics C ystein S N F e 3+ N N N H O CHEMICAL REVIEWS Volume 104, Issue 9 (September 8, 2004) 3947-3980 Mechanism of Oxidation Reactions Catalyzed by Cytochrome P450 Enzymes Bernard Meunier, Samuël P. de Visser, and Sason Shaik <http://dx.doi.org/10.1021/cr020443g>http://dx.doi.org/10.1021/cr020443g H F la v o p ro tein NAD o x . fo rm FM NH 2 red . fo rm F la v o p ro tein FM N o x. form NADH red . fo rm H em e p ro tein F e 3+ S u b strate-red . form o x . fo rm H em e p ro tein F e 2+ S u b stra te-o x.fo rm red . fo rm F lavin m on on u cleotid e N ico tin am id ad en in e d in u cleotid e O NH2 O N O O P HO N N N O P O OH NH2 N NAD H 3C N O P O OH N O OH HO NADH H 3C H N H 3C N O NH NH H OH H N H OH H OH H OH OH H OH H N FM N N N N OH N OPO3 P O O HO OH HO HO NH2 O O O OH HO H 3C NH2 N O O O H H O 2- OPO3 O 2- FM NH2 CYP450 families and sub-families Family 1: CYP1A1 Aromatic hydrocarbon hydroxylase, metabol. PAH etc. OH O OH Nu M o re h yd ro p h ilic n o t tox. OH Nu CYP1A2 Ox of arylamines, nitrosamines, aromatic hydrocarbons Family 2: Family 3: CYP2A6 CYP3A4 CYP2B6 CYP2C CYP2D6: Often enantioselective, lipophil. amines CYP2E1: Halogenated hydrocarbons, other org solvents CYP450 / Mechanisms of metobolic transformations Hydroxylation of alkane Dehydrogenation of alkane R -O -H R -H + Fe H 2O 3+ C Y P 450 F e 3+ F e 4+ O H F e 3+ O H H C Y P 450 C Y P 450 C Y P 450 R R -H NA DPH R = H F e 5+ O C Y P 450 F e2+ R -H C Y P 450 R -H H 2O O2 2H F e3+ O2 F e2+ C Y P 450 O2 R -H C Y P 450 e F e3+ O2 C Y P 450 R -C H 3 C Y P 450 O ther enzym es R -C H 2 O H R -C O 2 H R -H R -H A llylic alch oh ol Proposed mech. ox of alkenes F e 3+ OH R ad ical -com p lex A lk en e, arom atic -com p lex H R R R F e4+ O O Fe Fe 5+ 3+ O O 1,2 m igration R alkynes R O R O F e3+ O F e 3+ R R F e 3+ O H 2O R O O R OH Proposed mech. ox of aromatics R O R F e3+ F e 3+ R R R R R F e4+ O R taut R=H F e3+ O O F e 5+ O F e3+ H H HO Proposed mech. react. on heteroatom cont. compounds H O H X X R R Fe 5+ O Fe 4+ X X F e4+ O H i.e. N oxid e R R F e3+ OH F e 3+ OH O + H X -R X R i.e. h em iacetal N, O, S dealkylation cleav. of small alkylgroups (Me) Dehalogenation: HX + carbonyl comp. Proposed mech. react. on heteroatom cont. compounds H O H N N R N N R R R sulfide ox, see FMO N -oxid e Fe 5+ O Fe 4+ F e4+ O H F e 3+ OH O R '' N R S tab le if R , R ' R ''° H R' H OH O H N R' O OH H N H R earrang. N R N O R R' R' h yd roxylam in e O H N n itroso com p . h em iam in al H N R R' CYP450 Induction / inhibition by xenobiotics Xenobiotics may enhance metabol. of them selvs as well as other comp. taken at the same time Induce transcript CYP450 mRNA - Synth. CYP450 enzymes (enzyme induction) •Drugs •Ethanol •Organic solvents •Components in cig. smoke • OH N H OH O OH R HO R=H: H y p e ric in HO R=OH P s e u d o h y p e ric in St. Johns Worth (Johannesurt, prikkperikum) OH O OH CYP450 Inhibitors Reversible CYP enzyme inhibitors: Several drugs ex. antimycotic azoles S q u a le n e e p o k s id s y k la s e S q u a le n e e p o k s id a s e HO O S q u a le n e A n tim y c o tic a lly la m in e s H L a n o s te ro l C Y P 4 5 0 : L a n o s te ro l 1 4 a -d e m e ty la s e K lo trim a z o le C a n e s te n e tc A n tim y c o tic a zo le s N N Cl HO HO E rg o s te ro l H C e ll w a ll c o m p o n e n t fu n g i H H HO K o le s te ro l H CYP450 Inhibitors Complexation inhibitors ex. metabolites from alkylamines C YP 450 R -N M e 2 A lk y la m in e N o in h ib . a c tiv ity R -N = O N itro s o a lk a n e Irre v e rs ib le c o m p le x a tio n w ith F e (II) h e m e in C Y P s u b fa m ilie s Mechanism based inhibitors (suicide inhib) ex. alkynes O H 2O R F e O 3+ R R R O C Y P 45 0 OH H H H HO E th y n y l e stra d io l OH O N H 2 -E n zym e R N H E n zym e Phase 1 react. not involving CYP450 Other microsomal enzymes Azoreductase O O H 2N H 2N S N N O H 2N S NH2 P ron tocil NH2 O Su lfanilam ide Nitroreductase R NO2 R NH2 Flavinmonooxygenase-FMO (N and S-ox.) Peroxidases microsome: Artefactual spherical particle, not present in the living cell, derived from pieces of the endoplasmic reticulum present in homogenates of tissues or cells: microsomes sediment from such homogenates when centrifuged at 100 000 g and higher: the microsomal fraction obtained in this way is often used as a source of mono-oxygenase enzymes. Flavinmonooxygenase-FMO Cont. FAD F la v in m on on u cleotid e F la vin ad en in e d in u cleotid e O H 3C N H 3C NH N N H OH H OH H OH O NH2 N O O P O OH N O NH N R N O N HO N N N O P O OH P O N OR O P O OH NAD OH H OH H OH H OH H OH 2- OPO3 N H 3C N N NH N H OH H OH H OH OH HO A d en in e (V it. B 4) N O OR N O HO O N N N N O H 3C N icotin ic acid / N iacin (V it. B 3) HO C O 2H NADH O 2- FM N H 2 NH2 N R = P h o sp h ate: N A D P + , N A D P H N NH H OH N O H 3C O H O NH2 HO R=H OH HO HO NH2 O O O O H N N H OH N R ib oflavin (V it B 2) NH2 OH H 3C FM N OH H H O P N NH OPO3 NH2 O H 3C N O HO N O N ico tin am id ad en in e d in u cleo tid e O H 3C OH HO FAD H 3C O N O O H N FA D H 2 N O H 3C Flavinmonooxygenase-FMO Ox of soft Nu O R H P eroxide P eracid S u b strate + O Nu O ROH N -oxid e Su lfoxid e Nu S u b strate-O OH O H 3C H N H 3C N O NH N O H 3C H N H 3C N OH O H 2O NH N O O H 3C N H 3C N R R R FA D H 3C H 3C O2 H N N R O NH N H FADH2 •Amine: ox. to N-oxide / hydroxylamine •Sulfide: ox to sulfoxide , furter to sulfone O •Thiol: R -SH R -S-S -R R -S -S-R N A D PH O N A D P+ NH N O Non-microsomal enzymes •Enzymes in mitokondria •Enzymes in soubile tissue fractions A lch o h o l d eh y d ro g en a se (N A D co n t.) a ld eh y d e / k eto n e R -O H O A ldeh yde d ehydrogenase O R R OH H p rim o r sec. A ldeh yde d ehyd rogenase A lcohol deh ydrogen ase C H 2O C H 3O H H C O 2H rel. fast Slow EtO H com etitive substr. T ox. effects: A cidosis B lind ness D ietary folic acid O O HN O N H H N N H H C O 2H C O 2H N H N H O O C O 2H HN O T etrahyd rofolate N H N H H N N H N H O 10-F orm yl-T H F deh ydrogen ase C O 2H C O 2H CO2 Non-microsomal enzymes (Phase 1) Molybdenum Hydroxylases •Aldehyde oxidase •Xantine oxidase •Xantine dehydrogenase Xanthine oxidase Electron transfer: Cont. Mo in cat. site Cont FAD and 2 Fe/s clusters Use H2O not O2 FAD - Fe2S2ˇI - Fe2S2ˇII - Moco - Substrate Active form •Aldehyde oxidase O R O H R N N OH H 2N O A ld ehyd e oxid ase E sterase / hyd rolysis N N H 2N N N H N HO AcO OH N HO A cO N N HN O F am ciclovir A n tiviral (H erpes etc) P ro-drug A za h eterocycle •Xantine oxidase xanthine oxidoreductase •Xantine dehydrogenase O O (requires NAD+) N N HN O HN N N H O N H HN N H O X anth in e H ypoxan thin e O HN N N N H A llop urin ol HN O N N H H N O N H N H U ric acid T reatm ent of gout (p odagra) O P enciclovir N H A lloxanth in e Inh ib. enzym e Non-microsomal enzymes (Phase 1) Oxidative deamination of amines •Monoamine oxidase (MAO) •Diamine oxidase (DAO) R-CH 2 -NH 2 + O2 O NH 2 HO MAO R-CHO [R-CH=NH] HO N H N H Serotonin 5-Hydroks otryptamin (5-HT) + Serotonine: •Neurotransmittor; temp. control, mood •Depresion: Low serotonine activity •MAO Inhibitors - Older antidepresants (low selectivity) Moklobemid Aurorix® Moklobemid® O O N N H Cl O th e r M A O s u b s tra te s : OH HO HO H N N o t M A O s u b s tra te s (s u b s t a t -C ): HO H NH2 NH2 HO N o ra d re n a lin e D o p a m in e (S )-a m fe ta m in Low dopamine conc. ≈ Parkinston NH 4 + Non-selective monoamine re-uptake inhib. Tricyclic antidepressants SSRI (selective serotonine re-uptake inhib.) “Lykkepiller” Prozac etc (Fontex) -Arylam X H2 N Active transport re-uptake transmittor (not Acetylcholine) - -Arylamin Y R Z H O F3 C N Stor gruppe / sterisk hinding Hindrer reopptak -Arylaminer HO HO HO OH NH2 DA HO HO NH 2 NH2 NE N H 5-HT N Non-microsomal enzymes (Phase 1) Oxidative deamination of amines •Monoamine oxidase (MAO) •Diamine oxidase (DAO) HN Oxidize diamines, histamine N NH2 MAO like enzymes in plants NH2 HN N N N N H N N N BAP C Y to k in in (P la n t g ro w th h o rm o n e ) N H N N + N O N H N N N N H N N N C K a n a lo g s , A n d e rs B rå th e N H Non-microsomal enzymes (Phase 1) Miscellaneous react. Reductions R S S Hydrolysis - Esterases R R -S H O R -S O -R R -S -R (i.e .D M S O ) R O R' O R -C O -R R -C H O H -R R -N O -R R -N H -R R O R N OH R' R '' R -C H = C H -R R -C H 2 -C H 2 -R R -O H R -H A r-O H A r-H Esters as pro-drugs -Oxidation R -C H 2 -C H 2 -C O 2 H R -C H 2 -C H 2 -C O -S -C o A R -C O 2 H + NH2 C H a -C O -S -C o A N N A c etyl-C o A O S H N OH H N O O O O O P P O O O N N O O Nu PO3 A c e ty l-C o A OH R -C O -S -C oA Pathways of metabolism Phase 1: Biotransformation Attachment of new functional groups, transformation of exist. funct. groups oxidation, reduction, hydroxylation, hydrolysis etc. Phase 2: Conjugation. Masking of an exist. funct. group by for instance acetylation, glycosylation, attachment amino acid etc More hydrophilic drug Renal excretion N CH3 NH NH C o n ju g a tio n g lu cu ro n ic a cid D e m eth y la tio n HO O M o rp h in e OH P h a se 1 HO O OH P h a se 2 HO O OH HO O O OH C O 2H Phase 2: Conjugation Most comp. excreted as cojugates, ionic, hydrophilic groups added, most common glucuronation •Glucuronic acid conjugation •Sulfate conjugation •Conjugation with amino acids •Acetylation •Glutathione conjugation •Methylation Phase 2: Conjugation •Glucuronic acid conjugation Substrates: RXH: Xenobiotic / Phase 1 metabolite •Alchohols •Phenols X C O 2H HO OH •Sulfides O N O O OH OH •Amines R H OH OH G lu c o s e OH O P O OH P N O C O 2H X O OH R OH OH U D P -G lu c u ro n a te HO OH •Carboxylic acids •1,3-Dicarbonyls RXH C O 2H X O OH R K id n e y U riv e e x c re tio n OH OH OH OH P o o r re a b s o rb . B ile (g a lle ) R e a b s o rb Entro-hepatic recycling Important for many hormones etc RXH C O 2H X O OH C O 2H X O OH OH OH R In te s tin e R O O H y d ro ly sis H R O O O H C O 2H R C O 2H O O OH O OH OH O OH P P U rid in e R HNu OH M a c ro m o le c u le OH U D P -G lu c u ro n a te R e la tiv e ly la b ile A c y l m ig r. Nu M a c ro m o le c u le R O A lte re d p ro te in (h a p te n ) U n w a n te d im m u n e re s p o n c e / a lle rg ic re a c t. e x . N S A ID s U rin e pH <7 Phase 1 A r-N H 2 A r-N H -O H A r-N H -O H -G lu A r-N A r-N H -O H H 2O N itre n e (c .f. c a rb e n e ) B la d d e r c a n c e r Phase 2: Conjugation •Sulfate conjugation: Phenols, (alcohols, N-compounds) NH2 N O ATP O O O N N S O P HO O O O OH H O 3P O S N O OH PAPS •Conjugation with amino acids (Most ofthen Gly): Carboxylic acids O R -C O 2 H R H 2N S C oA O C O 2H R N H C O 2H No tox. conjugates known Phase 2: Conjugation •Acetylation: N-compounds O H 3C NH S O C oA N •Glutathione conjugation: Electrophilic species •Alkylhalides •Epoxides •Michael acceptors etc O R -X + HS O C O 2H N H S N H HN HN H S -R SR OH H 2O O M e rca p tu ric a cid d e r. O O N OH HN NH2 O G lu ta th io n e c o n ju g a te O P hase 1 C Y P450 S C O 2H NH2 O HN O R HN HN O C O 2H R C O 2H may otherwise alkylate biomolecules O O H SR OH P a ra c e ta m o l Br H 2N L ive r P ro te in S RSH S Br R Br L iv e r d a m m a g e Nu (i.e . D N A ) Phase 2: Conjugation •Methylation (O and N- compd) Prod. may be more lipophilic React. mainly aimed at converting endogenic compouds O.Metylation by COMT (catecol O-methyl transferase) HO C O 2H H 2N NH2 N N N ATP S O H 3C HO HO HO N OH S A M (S -a d e n o c y l m e th io n in e ) SAM may also methylate N-comp. O H 3C OH HO OH NH2 MAO HO HO OH CHO A ld e h yd e D e h yd ro g e n a s e HO HO C O 2H HO N o ra d re n a lin e (N o re p in e p h rin e ) COMT COMT OH OH H 3C O MAO NH2 A ld e h y d e D e h y d ro g e n a se H 3C O HO HO OH NH2 E p h e d rin e A d re n e rg a g o n is t C O 2H










