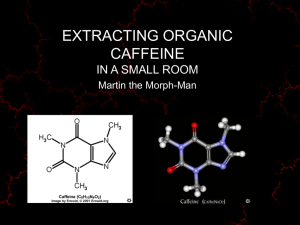
organic-compounds
advertisement

DO NOW 1-6-15 Directions: Write the question and answer. 1. The consumers at the top of the food pyramid have much less energy available to support them than those closer to the bottom. What causes this to occur? A. Energy at each level decreases as it is used by organisms at that level. B. Tertiary consumers eat all the producers in the pyramid. C. Tertiary consumers eat all the primary consumers in the pyramid. D. Energy at each level is hidden by animals that occupy that level. 2. An abundance of ___________ is important in the stability of an energy pyramid. A. producers B. 1st level consumers C. carnivores D. 2nd level consumers DO NOW 1-7-15 Directions: Write the question and answer. 1. A compound is organic if the carbon is bonded to at least one _____. a. hydrogen b. phosphorous c. oxygen d. sulfur 2. Organic compounds contain carbon, hydrogen, and other elements such as – A. aluminum and nickel B. sodium and chlorine C. nitrogen and sulfur D. magnesium and calcium DO NOW 1-8-15 1. Which formula represents an organic compound? A. NH3 B. H2O C. C6H12O6 D. AgNO3 2. The compounds carbon dioxide (CO2) and methane (CH4) both contains carbon atoms, but carbon dioxide is not an organic compound. Why? A. organic compounds do not include oxygen elements B. organic compounds cannot be gases C. organic compounds are only found in nonliving things D. organic compounds must have carbon and hydrogen atoms bonded together Organic Compounds By: Sabin Thomas EQ •What elements are common in our body? What is an element and compound? • An element is made up of one kind of atom and is represented by the symbol for the atom (EXAMPLE: O is the symbol for the element oxygen) • A compound is made of two or more kinds of atoms (EXAMPLE: H2O is the chemical formula for water) MISCONCEPTION NOTE!! • Not all elements have one letter for a symbol. Some elements have two-letter symbols, but the second letter will always be lower-case For ex: Fe is the symbol for iron Ca is the symbol for calcium • When a compound is made, the number of capital letters will tell you how many elements make it up. For ex. CO2 – has 2 element, carbon and oxygen NaCl – has 2 elements, sodium and chlorine CaCO3 – has 3 elements, calcium, carbon, and What does “organic” mean? • In the non-science world “organic” means something made from nature • In chemistry, “organic” means a carbon containing compound whose behavior and characteristics are a direct result of the flexibility of the carbon atom Organic Compound • Organic compounds are chemical compounds made of carbon and hydrogen atoms, and can include oxygen, nitrogen, phosphorous, or sulfur. • Ex. C6H6 – Benzene C8H10N4O2 – Cholesterol C10H12N3O3PS2 - Azinphos-methyl Think-Pair-Share • Where are organic compounds found? • Organic compounds are found in living or once living organisms Why is carbon so basic to life? • The reason is carbon’s ability to form stable bonds with many elements, including itself. This property allows carbon to form a huge variety of very large and complex molecules. DOL 1-6-15 • 1. What is a compound made of ? • A. one element B. two or more elements • C. One compound D. two or more compounds • • • • • Identify which is an element or compound 2. O2 3. Co 4. C8H10N4O2 5. MgO DOL 1-6-15 1. What is a compound made of ? A. one element B. two or more elements C. One compound D. two or more compounds 2. What makes a compound organic? A. hydrogen and oxygen B. oxygen and carbon C. carbon and hydrogen D. carbon and silver 3. Where are organic compounds found in nature? A. In non-living things B. In metals C. In living or once living things D. All of the above Types of Organic Compounds The millions of organic compounds can be grouped into just four major types: • Proteins • Nucleic Acids • Carbohydrates • Lipids ALL OF WHICH ARE FOUND IN LIVING THINGS!! Proteins • Are made up of carbon, hydrogen, oxygen, nitrogen, and sometimes sulfur atoms • Are most abundant organic compounds in living cells • Are essential in building and repairing body cells and tissues • Some foods including fish, eggs, meat, nuts, and beans are high in protein Nucleic Acids • Are mostly made of carbon, hydrogen, oxygen, nitrogen, and phosphorous atom • The two kinds of nucleic acids are DNA (deoxyribonucleic acid) and RNA (ribonucleic acid). • DNA is the genetic material that carries information about an organism. • RNA is important for making proteins. Carbohydrates • Consists mostly of carbon, hydrogen, and oxygen atoms where the proportion of H atoms to O atoms is 2:1. • Are the chief energy sources in all organisms • Provides raw fuel for muscles and organs • A lot of food that comes from plants have starches in them. Your body breaks down the starches into a sugar that your cells can use for energy. Lipids • They are made of mostly carbon and hydrogen atoms • Includes fats and fat molecules • Come from a combination of glycerol and fatty acids • Stores energy • Insulators to protect from cold • Fatty acids – come from nuts, meat, and milk Elements Found in Organic Compounds Use the following acronym to remember the elements found in organic compounds: N-CHOPS Nitrogen Carbon Hydrogen Oxygen Phosphorous Sulfur Can you identify the Organic Compounds from this list? 1. C6H8O6 – Ascorbic Acid (vitamin C) 2. CO2 – Carbon Dioxide 3. HCl – Hydrochloric Acid 4. C8H10N4O2 – Caffeine 5. C27H46O – Cholesterol 6. H2O2 – Hydrogen peroxide 7. C19H19N7O6 – Folic Acid (Vitamin M) 8. C6H12O6 – Glucose 9. MgO – magnesium oxide 10. O3 – Ozone 11. C13H18O2 – Ibuprofen 12. C20H30O – Retinol (Vitamin A) The Organic Compounds Are… 1. C6H8O6 – Ascorbic Acid (vitamin C) 2. CO2 – Carbon Dioxide 3. HCl – Hydrochloric Acid 4. C8H10N4O2 – Caffeine 5. C27H46O – Cholesterol 6. H2O2 – Hydrogen peroxide 7. C19H19N7O6 – Folic Acid (Vitamin M) 8. C6H12O6 – Glucose 9. MgO – magnesium oxide 10. O3 – Ozone 11. C13H18O2 – Ibuprofen 12. C20H30O – Retinol (Vitamin A) DOL 1-7-14 1. KOH – Potassium Hydroxide 2. NaCl – Sodium Chloride (table salt) 3. C19H28O2 – Testosterone 4. CH4N2O – Urea 5. H20 – Water 6. C12H22O11 – Sucrose (table sugar) 7. CO – carbon monoxide The Organic Compounds Are… 1. KOH – Potassium Hydroxide 2. NaCl – Sodium Chloride (table salt) 3. C19H28O2 – Testosterone 4. CH4N2O – Urea 5. H20 – Water 6. C12H22O11 – Sucrose (table sugar) 7. CO – carbon monoxide