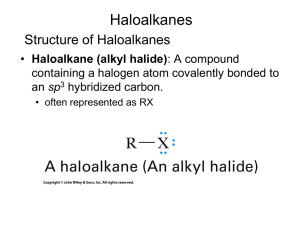11. Reactions of Alkyl Halides: Nucleophilic Substitutions and
advertisement

11. Reactions of Alkyl
Halides: Nucleophilic
Substitutions and
Eliminations
Based on McMurry’s Organic
Chemistry, 7th edition
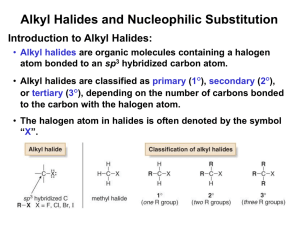
Alkyl Halides React with
Nucleophiles and Bases
Alkyl halides are polarized at the
carbon-halide bond, making the carbon
electrophilic
Nucleophiles will replace the halide in
C-X bonds of many alkyl halides
(reaction as Lewis base)
2
Alkyl Halides React with
Nucleophiles and Bases
Nucleophiles
that are Brønsted
bases produce elimination
3
Substitution vs. Elimination
4
The Nature of Substitution
Substitution requires that a "leaving group", which is
also a Lewis base, departs from the reacting molecule.
A nucleophile is a reactant that can be expected to
participate as a Lewis base in a substitution reaction.
5
11.1 The Discovery of the Walden
Inversion
In 1896, Paul Walden showed that (-)-malic acid could
be converted to (+)-malic acid by a series of chemical
steps with achiral reagents
This established that optical rotation was directly related
to chirality and that it changes with chemical alteration
Reaction of (-)-malic acid with PCl5 gives (+)-chlorosuccinic acid
Further reaction with wet silver oxide gives (+)-malic acid
The reaction series starting with (+) malic acid gives (-) acid
6
The Walden Inversion (1896)
7
O
O
HO
PCl5
OH
H
O
HO
OH
ether
(retention)
OH
H
O
R(-)-Malic acid
[]D= -2.3o
Cl
R(-)-Chlorosuccinic acid
Ag2O,
Ag2O,
H2O
H2O
(inversion)
(inversion)
O
O
PCl5
HO
OH
O
Cl
H
S(+)-Chlorosuccinic acid
ether
(retention)
HO
OH
O
HO
H
S(+)-Malic acid
[]D= +2.3o
8
Significance of the Walden
Inversion
The
reactions involve
substitution at the chiral center
Therefore, nucleophilic
substitution can invert the
configuration at a chirality
center
9
11.2 Stereochemistry of
Nucleophilic Substitution
A more rigorous Walden
cycle using 1-phenyl-2propanol (Kenyon and
Phillips, 1929)
Only the second and fifth
steps are reactions at
carbon
Inversion must occur in
the substitution step
10
11
The inversion step (step 2):
12
Two Stereochemical Modes of
Substitution
Substitution with
inversion:
H3C
X
R
CH3
OH-1
+ X-1
HO
H
H
R
Substitution with retention: (Note: if both occur
simultaneously, the result is racemization)
H3C
X
R
H
OH-1
H3C
OH + X-1
R
H
13
Hughes’ Proof of Inversion
React S-2-iodo-octane
with radioactive iodide
Observe that
racemization (loss of
optical activity) of
mixture is twice as fast
as incorporation of label
Racemization in one
reaction step would
occur at same rate as
incorporation
14
Hughes’ Proof of Inversion
inversion
H
I
I
H
two molecules of
racemic mixture
}
no reaction
H
H
I
I
racemization
H
I
{
I
H
}
two molecules of
racemic mixture
racemization
H
I
H
I
15
Substitution Mechanisms
SN1
Two
steps with carbocation intermediate
Occurs in 3°, allyl, benzyl
SN2
Concerted
mechanism - without intermediate
Occurs in primary, secondary
16
11.3 Kinetics of Nucleophilic
Substitution
Rate is the change in concentration with
time
Depends on concentration(s),
temperature, inherent nature of reaction
(energy of activation)
A rate law describes the relationship
between the concentration of reactants
and the overall rate of the reaction
A rate constant (k) is the proportionality
factor between concentration and rate
17
Kinetics of Nucleophilic Substitution
Rate = d[CH3Br]/dt = k[CH3Br][OH-1]
This reaction is second order: two
concentrations appear in the rate law
SN2: Substitution Nucleophilic 2nd order
18
11.2 The SN2 Reaction
Reaction occurs with inversion at
reacting center
Follows second order reaction kinetics
Ingold nomenclature to describe ratedetermining step:
S=substitution
N
(subscript) = nucleophilic
2 = both nucleophile and substrate in
rate-determining step (bimolecular)
19
SN2
Process
20
SN2 Transition State
The transition state of
an SN2 reaction has a
planar arrangement of
the carbon atom and
the remaining three
groups
Hybridization is sp2
21
22
23
11.3 Characteristics of the SN2
Reaction
Sensitive to steric effects
Methyl halides are most reactive
Primary are next most reactive
Unhindered secondary halides react
under some conditions
Tertiary are unreactive by this path
No reaction at C=C (vinyl or aryl halides)
24
Reactant and Transition-state Energy
Levels Affect Rate
Higher reactant energy
level (red curve) = faster
reaction (smaller G‡).
Higher transitionstate energy level (red
curve) = slower
reaction (larger G‡).
25
26
Steric Effects on SN2 Reactions
The carbon atom in (a) bromomethane is readily accessible
resulting in a fast SN2 reaction. The carbon atoms in (b) bromoethane
(primary), (c) 2-bromopropane (secondary), and (d) 2-bromo-2-methylpropane
(tertiary) are successively more hindered, resulting in successively slower SN2
27
reactions.
Steric Effect in SN2
28
Steric Hindrance Raises
Transition State Energy
Very hindered
Steric effects destabilize transition states
Severe steric effects can also destabilize
ground state
29
Order of Reactivity in SN2
The more alkyl groups connected to the reacting
carbon, the slower the reaction
30
Vinyl and Aryl Halides:
31
Order of Reactivity in SN2
-1
R
Br + Cl
Br
ethyl
1.0
DMF
R
Br
propyl
0.69
Cl
+ Br-1
Br
isobutyl
0.33
Br
neopentyl
0.000006
32
The Nucleophile
Neutral or negatively charged Lewis base
Reaction increases coordination (adds a
new bond) at the nucleophile
Neutral
nucleophile acquires positive
charge
Anionic nucleophile becomes neutral
See Table 11-1 for an illustrative list
33
For example:
Br
CH2
Cl
C
+ CN-1
+ H2O
CH2
OH2
N
+ Br-1
+ Cl-1
34
35
Relative Reactivity of Nucleophiles
Depends on reaction and conditions
More basic nucleophiles react faster (for similar
structures. See Table 11-2)
Better nucleophiles are lower in a column of the
periodic table
Anions are usually more reactive than neutrals
36
37
The Leaving Group
A
good leaving group reduces the
energy of activation of a reaction
Stable anions that are weak bases
(conjugate bases of strong acids) are
usually excellent leaving groups
Stronger bases (conjugate bases of
weaker acids) are usually poorer
leaving groups
38
The Leaving Group
39
Poor Leaving Groups
If a group is very basic or very small, it does not
undergo nucleophilic substitution.
40
Converting a poor LG to a good LG:
41
The Solvent
Protic solvents (which can donate hydrogen bonds; -OH or –NH) slow
SN2 reactions by associating with reactants (anions).
Energy is required to break interactions between reactant and solvent
Polar aprotic solvents (no NH, OH, SH) form weaker interactions with
substrate and permit faster reaction
42
Some Polar Aprotic Solvents
O
H3C
H3C
CH3
N
S
CH3
O
dimethylsulfoxide
(DMSO)
CH3
N
C
H
N
CH3
N
H3C
O
P
CH3
CH3
CH3
hexamethylphosphoramide
(HMPT)
dimethylformamide
(DMF)
43
44
Summary of SN2
Characteristics:
Substrate: CH3->1o>2o>>3o (Steric effect)
Nucleophile: Strong, basic nucleophiles favor
the reaction
Leaving Groups: Good leaving groups (weak
bases) favor the reaction
Solvent: Aprotic solvents favor the reaction;
protic reactions slow it down by solvating the
nucleophile
Stereochemistry: 100% inversion
45
Prob. 11.37 Arrange in order of SN2
reactivity
46
11.4 The SN1 Reaction
Tertiary alkyl halides react rapidly in protic solvents by
a mechanism that involves departure of the leaving
group prior to the addition of the nucleophile.
Reaction occurs in two distinct steps, while SN2 occurs
in one step (concerted).
Rate-determining step is formation of carbocation:
47
SN1 Reactivity:
48
SN1 Energy Diagram
49
Rate-Limiting Step
The overall rate of a reaction is controlled by the
rate of the slowest step
The rate depends on the concentration of the
species and the rate constant of the step
The step with the largest energy of activation is
the rate-limiting or rate-determining step.
See Figure 11.9 – the same step is ratedetermining in both directions)
50
SN1 Energy Diagram
51
52
Stereochemistry of SN1
Reaction
The planar carbocation intermediate leads to loss of chirality
Product is racemic or has some inversion
53
54
Stereochemistry of SN1
Reaction
•Carbocation is usually biased to react on side
opposite leaving group because it is unsymmetrically
solvated
•The second step may occur with the carbocation
loosely associated with leaving group.
•The result is racemization with some inversion:
55
Effects of Ion Pair Formation
56
Prob. 11.9: What is the %
inversion & racemization?
57
Prob. 11.9: What is the %
inversion & racemization?
Product is 9.9% optically pure, which rounds off to 55%
inverted, 45% retained.
There is 10% inversion accompanied by 90% racemization,
a typical SN1 result.
58
11.5 Characteristics of the SN1
Reaction
Tertiary alkyl halides are the most reactive
simple halides by this mechanism
Controlled by stability of carbocation
59
Relative Reactivity of Halides:
60
Delocalized Carbocations
Delocalization
of cationic charge
enhances stability
Primary allyl is more stable than
primary alkyl
Primary benzyl is more stable
than allyl
61
Allylic and Benzylic Halides
Allylic and benzylic intermediates stabilized by
delocalization of charge (See Figure 11-13)
Primary
allylic and benzylic are also more
reactive in the SN2 mechanism
62
63
Relative SN1 rates (formolysis):
RCl + HCOO-1
Cl
Cl
3550
1.0
Cl
Cl
0.5
5670
64
Formation of the allylic cation:
Cl
Cl
65
Effect of Leaving Group on SN1
Critically dependent on leaving group
Reactivity: the larger halides ions are better leaving
groups
In acid, OH of an alcohol is protonated and leaving
group is H2O, which is still less reactive than halide
p-Toluensulfonate (TosO-) is an excellent leaving group
66
Nucleophiles in SN1
Since nucleophilic addition occurs after
formation of carbocation, reaction rate is not
normally affected by nature or concentration of
nucleophile
67
68
Solvent Is Critical in SN1
The solvent stabilizes the carbocation, and also
stabilizes the associated transition state. This
controls the rate of the reaction.
Solvation of a
carbocation by water
69
Polar Solvents Promote
Ionization
Polar, protic and unreactive Lewis base solvents
facilitate formation of R+
Solvent polarity is measured as dielectric polarization
(P) (Table 11-3)
70
Effect of Solvent
71
Solvent Polarity
72
Effects of Solvent on Energies
Polar solvent stabilizes transition state and
intermediate more than reactant and product
73
Summary of SN1
Characteristics:
Substrate: Benzylic~allylic>3o >2o
Nucleophile: Does not affect reaction (although
strong bases promote elimination)
Leaving Groups: Good leaving groups (weak
bases) favor the reaction
Solvent: Polar solvents favor the reaction by
stabilizing the carbocation.
Stereochemistry: racemization (with some
inversion)
74
Prob. 11.36 Arrange in order of SN1
reactivity
75
Practice Problem 11.2: SN1 or SN2?
76
Problem 11.13: SN1 or SN2?
77
Biological Substitution Reactions
78
Biological Substitution Reactions
79
Biological Substitution Reactions
80
11.7 Alkyl Halides: Elimination
Elimination is an alternative pathway to substitution
Elimination is formally the opposite of addition, and
generates an alkene
It can compete with substitution and decrease yield,
especially for SN1 processes
81
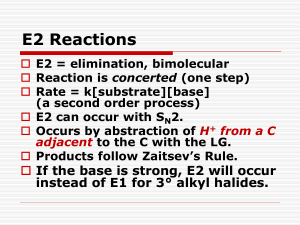
Zaitsev’s Rule for Elimination
Reactions (1875)
In the elimination of HX from an alkyl halide, the more
highly substituted alkene product predominates
82
Mechanisms of Elimination
Reactions
Ingold nomenclature: E – “elimination”
E1 (1st order): X- leaves first to generate a
carbocation
a base abstracts a proton from the
carbocation
E2 (2nd order): Concerted transfer of a proton to
a base and departure of leaving group
E1cb : Carbanion intermediate is formed in the
rate-determining step
83
E1 mechanism: starts out like SN1
84
E2 mechanism: concerted
85
E1cb: common in biochemical reactions
86
11.8 The E2 Reaction
Mechanism
A proton is transferred to base as leaving
group begins to depart
Transition state combines leaving of X and
transfer of H
Product alkene forms stereospecifically
87
88
E2 Reaction Kinetics
One
step (concerted): rate law
dependent on base and alkyl halide
Rate = k[R-X][B]
Reaction goes faster with stronger
base, better leaving group
89
Kinetic Isotope Effect
Substitute deuterium for hydrogen at
position
Effect on rate is kinetic isotope effect
(kH/kD = deuterium isotope effect)
Rate is reduced in E2 reaction
Heavier
isotope bond is slower to break
Shows C-H bond is broken in or before ratelimiting step
90
kH/kD
91
Geometry of Elimination – E2
Antiperiplanar allows orbital overlap and
minimizes steric interactions
92
E2 Stereochemistry
93
Comparison of SN2 and E2:
94
Predicting Product
E2 is stereospecific
Meso-1,2-dibromo-1,2-diphenylethane with base gives cis 1,2diphenyl-1-bromoethene
RR or SS 1,2-dibromo-1,2-diphenylethane gives trans 1,2-diphenyl1-bromoethene
95
Anti periplanar geometry
96
11.9 Elimination From
Cyclohexanes
Abstracted proton and leaving group
should align trans-diaxial to be anti
periplanar (app) in approaching transition
state (see Figures 11-19 and 11-20)
Equatorial groups are cannot be in proper
alignment
97
11.9 Elimination From
Cyclohexanes
98
Axial vs. Equatorial Leaving
Groups
99
100
11.10 The E1 Reaction
Competes with SN1 and E2 at 3° centers
Rate = k [RX]
101
102
Stereochemistry of E1
Reactions
E1 is not stereospecific and there is no
requirement for alignment
Product has Zaitsev orientation because the
step that controls product formation is loss of
proton after formation of carbocation
103
Comparing E1 and E2
Strong
base is needed for E2 but not
for E1
E2 is stereospecifc, E1 is not
E1 gives Zaitsev orientation; E2 may
not due to stereospecificity
E1 is favored in protic solvents;
competes with SN1
104
Comparing E1 and E2
105
E1cb:
106
A biochemical example (from fat
biosynthesis):
107
Reactivity Summary: SN1, SN2, E1, E2
108
General Pattern by Substrate
109
Primary alkyl halides (SN2 vs E2)
110
Secondary alkyl halides (SN2 vs E2)
111
Tertiary alkyl halides (SN1/E1 vs
E2)
112
Practice Problem 11.5
113
Answers
114
Problem 11.20
115
Problem 11.45: This halide does not
undergo SN1 or SN2 reactions. Why?
116
It also fails to eliminate HBr under
basic conditions. Why?
117