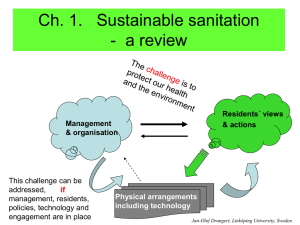
ppt - Sustainable Sanitation

4.6 Physical, biological and chemical treatment processes
What compounds can be removed from wastewater?
How can Nature assist or react?
Chemical ~ coagultation/flocculation, adsorption, precipitation, UV-radiation
More than 2,000 persons
Treatment results for small and large water utilities
Less than 2,000 persons
J-O Drangert, Linköping University, Sweden
B: Physical processes
screening flotation and sedimentation filtration forced microfiltration
Possible combinations of physical processes
Jan-Olof Drangert, Linköping university, Sweden
Screening of debris and other solid items
Solids trapped by a screen in a city wastewater treatment plant
Organics from kitchen pipe sorted out in a plastic screen
Jan-Olof Drangert, Linköping university, Sweden
Inlet of wastewater
Flotation and sedimentation processes
Inspection hole
Baffels
Outlet of treated water
Floating grease, particles, organisms
Jan-Olof Drangert, Linköping university, Sweden
Filtration – mainly by gravity
Partially unsaturated flow Saturated flow of wastewater
Jan-Olof Drangert, Linköping university, Sweden
Applied pressure
Forced micro-filtration
Manufactured porous material
Direction of filtered water flow
Jan-Olof Drangert, Linköping university, Sweden
C: Chemical processes
Adsorption of charged particles
Adsorption of phosphate on ferric hydroxide
OH
H
2
PO
4
- + Fe
OH
OH
H
2
PO
4
- + Al
OH
Adsorption of phosphate on aluminium hydroxide particles
G. Jacks, Royal Institute of Technology, Stockholm
Adsorption of charged particles to soil medium
The three important kinds of charged soil particles are :
1. Organic matter
RCOOH < > RCOO
-
+ H
+
(a negative pH-dependent charge)
R is phenolic ring derived from lignite in residues of plants
2. Clay minerals
Clay mineral consist of Al-Si-sheets with different cations (Na
+
, K
+ etc.) in between the sheets. There is a negative charge on sides and edges:
R-COO
-
R-COO
-
Pb
2+
Mineral grain
Organic ”overcoat” on a soil mineral
-
K
+
Cu
2+
K
+
-
Mg
2+
-
-
OH
Fe(III) + HAsO
4
-
OH
3. Ferric hydroxides
Fe(OH)
3
< > Fe(OH)
2-
+ H
+
(a pH-dependent positive charge)
G Jacks, Royal Institute of Technology, Stockholm
Adsorption of chemical compounds differ
Copper (Cu) and Zink (Zn) are positively charged, and adsorb easily on organic matter and clays when the pH > 7
Arsenic (As) is negatively charged and adsorbs easily on ferric hydroxides when pH < 7
G Jacks, Royal Institute of Technology, Stockholm
Precipitation and flocculation
• Precipitation – a chemical reaction between dissolved compounds to form solids
• Flocculation - an aggregation process (or processes) leading to the formation of larger particles from smaller particles
- + + -
-
+
+
+
-
G. Jacks, Royal Institute of Technology, Stockholm
UV-radiation by sunlight
Inactivation of microorganisms by UVAradiation and increased temperature http://www.sodis.ch/Text2002/T-
TheMethod.htm
Source: Ubomba-Jaswa et al. 2009
Shallow ponds with a dense population of algae
More diffuse stratification
Vertical view of the pond
K Tonderski, Linköping University Sweden
Strong algal stratification
Courtesy of Duncan Mara, University of Leeds, UK
Ozonation and chlorination
D: Biological processes
Karin Tonderski, Linköping university, Sweden
Biological processes - with air
Oxygen is vital for most living organisms, including bacteria and viruses. When oxygen is present, organic matter (measured as
BOD) is efficiently decomposed by organisms into CO
2
+ water:
Unsaturated soil profile
Organic matter
+ oxygen Aerobic bacteria
Jan-Olof Drangert,
Linköping university, Sweden
Biological processes - without air
Many microorganisms can survive in environments with no oxygen and they use other compounds for their survival:
Organic matter in wastewater
+ e.g. nitrate, sulphate or iron ions ( Fe 3+ )
Anaerobic microorganisms
Saturated soil profile with little or no oxygen
CO
2
+ e.g.
N
2
, S 2, Fe 2+
Jan-Olof Drangert,
Linköping university, Sweden
Microorganisms attached to surfaces are more stable than those suspended in water
Grain particle
Jan-Olof Drangert, Linköping university, Sweden
“
Redox-ladder
”
When microorganisms descend the redox-ladder they first use O
2 as an electron acceptor, then nitrate NO
3
, and further down other compounds as electron acceptors. The blue arrow indicates a reaction with energy-rich organic substances (electron donors) in the wastewater
O
2
H
2
O (oxygenisation)
NO
3
-
MnO
2
N
2
, N
2
O (denitrification)
Mn 2+
Fe 2+ Fe(OH)
3
SO
4
2-
CO
2
H
2
S (sulphate-reduction)
CH
4
(methanogenesis)
Gunnar Jacks, Royal Institute of Technology, Stockholm
Changes in concentrations of electron acceptors when organic matter (TOC) decomposes
Gunnar Jacks, Royal Institute of Technology, Stockholm
What happens in the root zone?
O
2
, sugars, proteins, etc
Water, nutrients, heavy metals, gases (e.g. CO
2
)
Organic matter, O
2
,
NO
3
, SO
4
2,
CO
2 etc
Jan-Olof Drangert, Linköping university, Sweden
Predation on microorganisms stimulates decomposition
Courtesy of Frida Lögdberg, Linköping university
Soil organisms vary tremendously in size and numbers
A teaspoon soil ~ one gram
Microbial group
Example Size
(µm)
Numbers
(per gram soil)
Biomass
(g wet mass per m
2
soil)
30
– 300
Bacteria
Fungi
Protozoa
Nematodes
Pseudomonas 0.5
– 1.5
Mucor
Euglena
Pratylenchus 1000
10
8
- 10
9
8 (hyphae 10
5 – 10 6 diameter)
15 * 50 10
3
- 10
5
10 – 10 2
50 - 500
0.5
0.1
– 20
– 10
Earthworms Lumbricus 100 000 1 - 100
Modified from Sylvia, D. et al . 2004. Principles and applications of soil microbiology
Organic matter is decomposed most efficiently in the top soil
10 6
Million organisms per gram soil
Anaerobic bacteria Aerobic bacteria
0 10 6
Soil surface
0.5 m
Courtesy of G. Jacks, Royal Institute of Technology, Stockholm