Nucleophilic Substitution (SN2) Reactions
advertisement

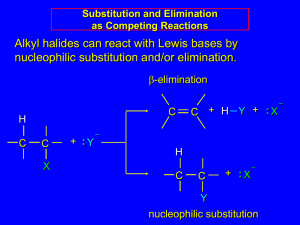
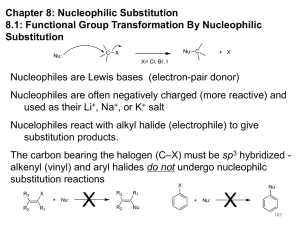
Chapter 8 I. Nucleophilic Substitution (in depth) II. Competion with Elimination QuickTime™ and a Sorenson Video decompressor are needed to see this picture. Nucleophilic Substitution Substrate is a sp3 hybridized carbon atom (cannot be an a vinylic halide or an aryl halide except under special conditions to be discussed in Chem 227) X C C X Kinetics Many nucleophilic substitutions follow a second-order rate law. CH3Br + HO – CH3OH + Br – rate = k [CH3Br] [HO – ] What is the reaction order of each starting material? What can you infer on a molecular level? What is the overall order of reaction? Bimolecular mechanism one step concerted HO – + CH3Br HOCH3 + Br – Bimolecular mechanism one step concerted HO – + CH3Br HOCH3 + Br – Bimolecular mechanism dHO dBr CH3 transition state one step concerted HO – + CH3Br HOCH3 + Br – Stereochemistry of SN2 Reactions Generalization QuickTime™ and a YUV420 codec decompressor are needed to see this picture. Nucleophilic substitutions that exhibit second-order kinetic behavior are stereospecific and proceed with inversion of configuration. Inversion of Configuration nucleophile attacks carbon from side opposite bond to the leaving group Inversion of Configuration nucleophile attacks carbon from side opposite bond to the leaving group three-dimensional arrangement of bonds in product is opposite to that of reactant Inversion of configuration (Walden inversion) in an SN2 reaction is due to “back side attack” P.Walden, Berichte, 29(1): 133-138 (1896) Riga Polytechnical College Could there be another mechanism that provides the same results? Roundabout SN2 Mechanism Traditional SN2 Mechanism Videos courtesy of William L. Hase, Texas Tech University SN2 Reaction Mechanisms: Gas Phase (2008) http://pubs.acs.org/cen/news/86/i02/8602notw1.html Traditional Physicist Roland Wester and his team in Matthias Weidemüller's group at the University of Freiburg, in Germany, in collaboration with William L. Hase's group at Texas Tech University, provide direct evidence for this mechanism in the gas phase. However, they also detected an additional, unexpected mechanism. In this new pathway, called the roundabout mechanism, chloride bumps into the methyl group and spins the entire methyl iodide molecule 360° before chloride substitution occurs. The team imaged SN2 reactions at different collision energies, which depend on the speed at which chloride smashes into methyl iodide. Data at lower collision energies support the traditional SN2 mechanism. However, at higher collision energies, about 10% of the iodide ions fell outside of the expected distribution. "We saw a group of iodide ions with a much slower velocity than the rest," says Wester. "Since energy is conserved, if iodide ions are slow, the energy has to be somewhere else." On the basis of calculations performed by their colleagues at Texas Tech, the team concluded that the energy missing from the iodide transfers to the methyl chloride product in the form of rotational excitation, supporting the proposed roundabout mechanism. Roundabout Fig. 1. Calculated MP2(fc)/ECP/aug-cc-pVDZ Born-Oppenheimer potential energy along the reaction coordinate g = RC-I - RC-Cl for the SN2 reaction Cl- + CH3I and obtained stationary points J. Mikosch et al., Science 319, 183 -186 (2008) Published by AAAS Fig. 2. (A to D) Center-of-mass images of the I- reaction product velocity from the reaction of Cl- with CH3I at four different relative collision energies J. Mikosch et al., Science 319, 183 -186 (2008) Published by AAAS Fig. 3. View of a typical trajectory for the indirect roundabout reaction mechanism at 1.9 eV that proceeds via CH3 rotation J. Mikosch et al., Science 319, 183 -186 (2008) Published by AAAS Stereospecific Reaction A stereospecific reaction is one in which stereoisomeric starting materials give stereoisomeric products. The reaction of 2-bromooctane with NaOH (in ethanol-water) is stereospecific. (+)-2-Bromooctane (–)-2-Octanol (–)-2-Bromooctane (+)-2-Octanol Stereospecific Reaction H (CH2)5CH3 CH3(CH2)5 H NaOH C Br CH3 (+)-2-Bromooctane HO C CH3 (–)-2-Octanol Question (+)-2-Bromooctane (–)-2-Octanol H (CH2)5CH3 CH3(CH2)5 H NaOH C Br HO C CH3 CH3 The absolute configurations of (+)-2-bromooctane and (–)-2-octanol are respectively: A) R- & R- B) S- and S- C) R- & S- D) S- & R- Answer (+)-2-Bromooctane (–)-2-Octanol H (CH2)5CH3 CH3(CH2)5 H NaOH C Br HO C CH3 CH3 The absolute configurations of (+)-2-bromooctane and (–)-2-octanol are respectively: A) R- & R- B) S- and S- C) R- & S- D) S- & R- 1) Draw the Fischer projection formula for (+)-S-2-bromooctane. 2) Write the Fischer projection of the (–)-2-octanol formed from it by nucleophilic substitution with inversion of configuration. CH3 H CH3 Br CH2(CH2)4CH3 HO H CH2(CH2)4CH3 R- Question True (A) / False (B) A racemic mixture of (R- ) and (S- )-2bromobutane produces an optically active product. Answer True (A) / False (B) A racemic mixture of (R- ) and (S- )-2bromobutane produces an optically active product. Optically inactive starting materials produce optically inactive products. The products in this case are also racemic. Inversion occurs with both enantiomers. A conceptual view of SN2 reactions Why does the nucleophile attack from the back side? Steric Effects in SN2 Reactions Crowding at the Reaction Site The rate of nucleophilic substitution by the SN2 mechanism is governed by steric effects. Crowding at the carbon that bears the leaving group slows the rate of bimolecular nucleophilic substitution. Reactivity toward substitution by the SN2 mechanism RBr + LiI RI + LiBr Alkyl bromide Class Relative rate CH3Br Methyl 221,000 CH3CH2Br Primary 1,350 (CH3)2CHBr Secondary 1 (CH3)3CBr Tertiary too small to measure A bulky substituent in the alkyl halide reduces the reactivity of the alkyl halide: steric hindrance Decreasing SN2 Reactivity CH3Br CH3CH2Br (CH3)2CHBr (CH3)3CBr Decreasing SN2 Reactivity CH3Br CH3CH2Br (CH3)2CHBr (CH3)3CBr Reaction coordinate diagrams for (a) the SN2 reaction of methyl bromide and (b) an SN2 reaction of a sterically hindered alkyl bromide Question Which chloride will react faster with NaI in acetone? A) B) C) D) Answer Which chloride will react faster with NaI in acetone? A) B) C) D) Crowding Adjacent to the Reaction Site The rate of nucleophilic substitution by the SN2 mechanism is governed by steric effects. Crowding at the carbon adjacent to the one that bears the leaving group also slows the rate of bimolecular nucleophilic substitution, but the effect is smaller. Effect of chain branching on rate of SN2 substitution RBr + LiI RI + LiBr Alkyl bromide Structure Relative rate Ethyl CH3CH2Br 1.0 Propyl CH3CH2CH2Br 0.8 Isobutyl (CH3)2CHCH2Br 0.036 Neopentyl (CH3)3CCH2Br 0.00002 Question Which alkyl chloride will react faster with NaI in acetone? A) B) C) D) Answer Which alkyl chloride will react faster with NaI in acetone? A) B) C) D) 8.1 Functional Group Transformation By Nucleophilic Substitution Nucleophilic Substitution – Y: + R X Y R + – :X nucleophile is a Lewis base (electron-pair donor) often negatively charged and used as Na+ or K+ salt substrate is usually an alkyl halide, (most often 1o) Nucleophiles The nucleophiles described in Sections 8.1-8.6 are anions. – .. – .. – .. – – : N3 : N C: : : HS HO CH O 3 .. .. .. But, all nucleophiles (neutral electron rich molecules) are Lewis bases. .. HOH .. .. CH3OH .. : NH3 Table 8.1 Examples of Nucleophilic Substitution Alkoxide ion as the nucleophile R' ..– O: .. + R X gives an ether R' .. O .. R + :X – Table 8.1 Examples of Nucleophilic Substitution Carboxylate ion as the nucleophile O R'C ..– O: .. + R X gives an ester O R'C .. O .. R + :X – Table 8.1 Examples of Nucleophilic Substitution Hydrogen sulfide ion as the nucleophile H ..– S: .. H .. S .. + R X gives a thiol R + :X – Question Select the major organic product when (S)-2propanol is reacted with SOCl2 in pyridine followed by the addition of NaSH in ethanol. A) B) C) D) Answer Select the major organic product when (S)-2propanol is reacted with SOCl2 in pyridine followed by the addition of NaSH in ethanol. A) B) C) D) Question The best combination of reactants for preparing (CH3)3CSCH3 is: A) (CH3)3CCl + CH3SK B) (CH3)3CBr + CH3SNa C) (CH3)3CSK + CH3OH D) (CH3)3CSNa + CH3Br Answer The best combination of reactants for preparing (CH3)3CSCH3 is: A) (CH3)3CCl + CH3SK B) (CH3)3CBr + CH3SNa C) (CH3)3CSK + CH3OH D) (CH3)3CSNa + CH3Br Table 8.1 Examples of Nucleophilic Substitution Cyanide ion as the nucleophile :N – C: + C R R X gives a nitrile :N + :X – Table 8.1 Examples of Nucleophilic Substitution Azide ion as the nucleophile – :N .. – : N .. + gives an alkyl azide + – :N N N .. .. R + N R + X :X – 8.2 Relative Reactivity of Halide Leaving Groups Generalization Reactivity of halide leaving groups in nucleophilic substitution is the same as for elimination. RI most reactive RBr RCl RF least reactive Problem 8.2 A single organic product was obtained when 1-bromo-3-chloropropane was allowed to react with one molar equivalent of sodium cyanide in aqueous ethanol. What was this product? BrCH2CH2CH2Cl + NaCN Br is a better leaving group than Cl Problem 8.2 A single organic product was obtained when 1-bromo-3-chloropropane was allowed to react with one molar equivalent of sodium cyanide in aqueous ethanol. What was this product? BrCH2CH2CH2Cl + NaCN :N C CH2CH2CH2Cl + NaBr Question What is the major product of the reaction of the dihalide at the right with 1 equivalent of NaSH in dimethyl sulfoxide? A) B) C) D) Question 8 What is the major product of the reaction of the dihalide at the right with 1 equivalent of NaSH in dimethyl sulfoxide? A) B) C) D) 8.12 Improved Leaving Groups Alkyl Sulfonates Leaving Groups We have seen numerous examples of nucleophilic substitution in which X in RX is a halogen. Halogen is not the only possible leaving group, though. Other RX Compounds O ROSCH3 O O ROS CH3 O Alkyl Alkyl methanesulfonate p-toluenesulfonate (mesylate) (tosylate) (triflate = -CF3 ) Behave in the same way as alkyl halides Preparation Tosylates are prepared by the reaction of alcohols with p-toluenesulfonyl chloride (usually in the presence of pyridine). ROH + CH3 SO2Cl pyridine O ROS O CH3 (abbreviated as ROTs) Tosylates Undergo Typical Nucleophilic Substitution Reactions H KCN H CH2OTs ethanolwater CH2CN (86%) The best leaving groups are weakly basic. Table 8.8 Approximate Relative Reactivity of Leaving Groups Leaving Relative Group F– Cl– Br– I– H 2O TsO– CF3SO2O– Conjugate acid pKa of Rate of leaving group 10-5 1 10 102 101 105 108 HF HCl HBr HI H 3 O+ TsOH CF3SO2OH conj. acid 3.5 -7 -9 -10 -1.7 -2.8 -6 Table 8.8 Approximate Relative Reactivity of Leaving Groups Leaving Relative Group Conjugate acid pKa of Rate of leaving group F– 10-5 HF Cl– 1 HCl Br– 10 HBr Sulfonate esters are extremely good – 2 I 10 HI sulfonate ions are very weakH bases. + H 2O 101 3O TsO– 105 TsOH CF3SO2O– 108 CF3SO2OH conj. acid 3.5 -7 leaving-9groups; -10 -1.7 -2.8 -6 Tosylates can be Converted to Alkyl Halides CH3CHCH2CH3 OTs NaBr DMSO CH3CHCH2CH3 Br (82%) Tosylate is a better leaving group than bromide. Tosylates Allow Control of Stereochemistry Preparation of tosylate does not affect any of the bonds to the chirality center, so configuration and optical purity of tosylate is the same as the alcohol from which it was formed. H H CH3(CH2)5 TsCl C CH3(CH2)5 C OH pyridine H3C H3C OTs Tosylates Allow Control of Stereochemistry Having a tosylate of known optical purity and absolute configuration then allows the preparation of other compounds of known configuration by SN2 processes. H H CH3(CH2)5 C Nu– OTs (CH2)5CH3 Nu C SN2 H3C CH3 Nucleophiles and Nucleophilicity Table 8.4 Nucleophilicity Rank Nucleophile strong good I-, HS-, RSBr-, HO-, RO-, CN-, N3NH3, Cl-, F-, RCO2H2O, ROH RCO2H fair weak very weak Relative rate >105 104 103 1 10-2 Table 8.4 Nucleophilicity Rank Nucleophile Relative rate good HO–, RO– 104 RCO2– 103 H2O, ROH 1 fair weak When the attacking atom is the same (oxygen in this case), nucleophilicity increases with increasing basicity. Nucleophiles and Nucleophilicity SN1 vs. SN2 Nucleophiles Many of the protic solvents in which nucleophilic substitutions can be carried out are themselves nucleophiles. .. HOH .. .. CH3OH .. and EtOH for example Solvolysis The term solvolysis refers to a nucleophilic substitution in which the nucleophile is the solvent. SN2 Reactions are favored in Polar Aprotic Non-nucleophilic Solvents An aprotic solvent is one that does not have an —OH group. SN1 Reactions are favored in Polar Protic Solvents Solvolysis Substitution by an anionic nucleophile: SN2 kinetics R—X + :Nu— R—Nu + :X— Solvolysis: SN1 kinetics + R—Nu—H + :X— R—X + :Nu—H Carbocation intemediate 2nd intermediate Solvolysis Substitution by an anionic nucleophile in an aprotic non-nucleophilic solvent SN2 kinetics R—X + :Nu— R—Nu + :X— Solvolysis (protic solvents) : SN1 kinetics R—X + :Nu—H products of overall reaction + R—Nu—H + :X— R—Nu + HX Example: Methanolysis Methanolysis is a nucleophilic substitution in which methanol acts as both the solvent and the nucleophile. CH3 CH3 CH3 R—X + : O: + R O: H H –H+ R O .. : The product is a methyl ether. Some typical solvents in solvolysis solvent product from RX water (HOH) methanol (CH3OH) ethanol (CH3CH2OH) ROH ROCH3 ROCH2CH3 O O formic acid (HCOH) O acetic acid (CH3COH) ROCH O ROCCH3 Question Which of the following is not a good nucleophile in an SN1 solvolysis reaction? A) NaOCH3 B) CH3OH C) CH3CH2OH D) H2O Answer Which of the following is not a good nucleophile in an SN1 solvolysis reaction? A) NaOCH3 B) CH3OH C) CH3CH2OH D) H2O