Inert Gas Purification Systems
advertisement

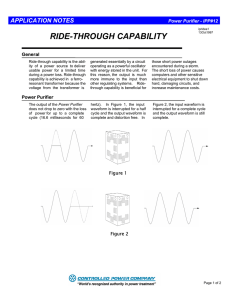
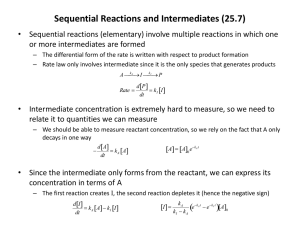
Inert Gas Purification Systems Why do we need them??? • Many of the materials used in research and development today are extremely air sensitive, and can be dangerous in the presence of air. • Purifiers are designed to remove oxygen, moisture and nitrogen/hydrogen from an inert gas to one part per million. • Safety is also a critical issue in experiments and research today. Purifier Components • • • • • • • • Circulation blower Copper catalyst Molecular Sieve Vacuum pump Solenoid Assembly Pressure control Regeneration control Manual footswitch • Isolation valves (automatic or manual) • Solvent removal systems • Titanium reactor (in the case of N2/H2 removal) • Exhaust traps Purifier theory The purpose of a purifier is to remove oxygen and water from an inert gas flowing through a controlled atmosphere system such as a glovebox. The typical purifier contains two purification agents. One agent is a molecular sieve that removes water by the process of molecular adsorption.The other agent is called Q5 and is an oxygen reactant material. Oxygen removal • Oxygen removal from argon, helium, or nitrogen is accomplished with a reactant/catalyst known as Q5, a material consisting of finely divided copper on an alumina matrix. The copper reacts with oxygen to form copper (II) oxide. Moisture removal • Moisture is removed by a molecular sieve enclosed in the same container as the oxygen reactant. Also removed by the molecular sieve at ambient temperatures are carbon dioxide, nitrogen dioxide, hydrogen sulfide, carbon monoxide and many organic compounds including: Alcohols, aromatics, amines, halogenated compounds, oxygenated compounds, hydrocarbons and organic acids. Regeneration Theory • To restore the purification capability of the material two reactions must take place. • The H2O trapped by the sieve must be removed completely. • The O2 must be removed from the Q5 reactant by reduction using hydrogen and heat. Regeneration theory (cont.) • The restoration of the sieve is accomplished by heating the material to vaporize the water. A dry gas is passed over the sieve that carries the water vapor out of the column. • Restoration of the the Q5 is accomplished by passing a H2 rich gas through the hot reactant. The H2 reacts with the saturated reactant to form metallic Cu and H20. The water is then pumped out. Materials that can damage the purifier • In general, any chemical that reacts with copper to form a more stable compound than copper (II) oxide will damage the oxygen reactant. • Volatile compounds containing sulfur or halogens are the most common of this type. • Also, alcohols, phosphenes, arsines, arsinate, and mercury vapor may also damage the oxygen reactant. If any of these materials are to be used in large quantities, a suitable trap should be installed. • Organic solvents will also damage the molecular sieve with long term exposure. Selecting a purifier • Consider ! • The size of the Glove-box • Determine the leak rates if applicable • Calculate the frequency of ante chamber operation • Determine the desired purity level Circulating Vs Purging • When purging a glove-box the atmosphere in the box can only achieve an O2 and H2O level that is present in the source gas. • Purging a box can be very expensive depending on the gas and the volume of the box to be purged. • When using a closed loop circulation purifier the supply gas does not need be pure. It is the job of the purifier to remove H2O and O2 in the gas to the <1ppm level. • The system only uses gas when transferring in and out of the box. Purifier options • Purifiers can be configured with dual columns for continuous operation. • They can be configured for different flow rates. • In addition to standard configurations custom solvent traps can be supplied for removal of harmful materials that can damage catalyst. • Nitrogen/hydrogen removal systems are also available. Utilities required • Two separate gases are required for standard purifiers. • Inert gas: Argon, Nitrogen or Helium with a delivery pressure of approx. 35psi • Regeneration gas: Your choice of a 4-5% mixture of H2 in argon or nitrogen. Helium is not recommended due to low molecular weight. • Electrical service: (varies depending on configuration) typically 115 VAC/20 A. • Venting: application specific Instrumentation • In order to determine the conditions of the atmosphere in the glove-box the following analyzers are available. • Moisture • Oxygen • Nitrogen • Other custom systems are available, i.e. GC/MS