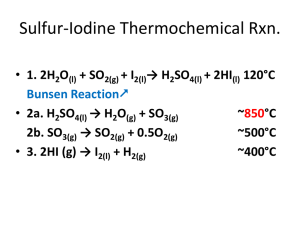
Reactor characteristics
advertisement

Reactors Reactors Reactor: a “container” where a reaction occurs Examples: Clear well at water treatment plant (chlorine contact) Activated sludge tank at wastewater treatment plant Treated wastewater discharge into a stream: stream = reactor Treated wastewater discharge into Cayuga lake: lake = reactor Gas tank leaking into soil: soil = reactor Reactor types What are your expectations? Feed Solution (glucose solution for pipe flow) Injection port C Flow with dispersion Peristaltic pump t or C Completely Mixed reactor t or reactors C Pipe flow reactor t Advection: mean flow C t = -u advection C x C What does it look like a short time later? x C x Dispersion: velocity fluctuations J D d Fick's first law Fick's second law C t What does it look like a short time later? C x = Dd dispersion C 2C x 2 x C x Reaction C C t = r = -kC reaction x What does it look like a short time later? C x Advection/Dispersion/Reaction C t = Dd total 2C x 2 -u C x C +r In three dimensions C t x D d 2C uC r C where + + x y z x Reactors: Closed vs. Open Closed: have little dispersion across the inlet and outlet boundaries Well defined reactor volume Examples tank with a small inlet and a small outlet __________________________________ lake ______ Open: have significant dispersion across the inlet and outlet boundaries Backmixing Example _______ river Reactors: Defining the Control Volume tracer Q Q Reactor Characterization Time scales hydraulic residence time average time for tracer to get from inlet to outlet Q “dead volume” t£ q dispersion upstream t ³ q “dead volume” t£ q ? flow rate t C ( t ) dt t= Open systems = volume Closed systems V 0 C (t )dt 0 Peclet Number Ratio of advection to dispersion how far does advection carry the fluid/width of tracer plume High L Pe Dd / U Peclet means primarily advection (_______________) plug flow Low Peclet means lots of mixing 2 2 Approximation for low Pe 2 t dispersion (Pe>10) Completely Mixed Flow Reactor Ft I H t K C C e 0 Closed reactor with no dead volume so theoretically t = . What is C0? How might you check this? Flow With Dispersion Equation C x ,t Solution ( x Ut ) 2 4 Dd t M e A 4Dd t for pulse mass input with advection and dispersion in only one direction Beware of units!!!! Adopt a consistent set! How can we get the dispersion coefficient? Estimating the Dispersion Coefficient Pe Pe 2 2 Approximation for Pe>10 t2 n 2 t i Ci t L Dd / U Definition of Pe t2 i 0 n C t i i 0 Dd LU Solve for Dd Pe Dd LU t2 2 2 Substitute approximation n t C t t i 0n i i C t i 0 i t 2 Mass conservation How much tracer comes out in 10 seconds? What are the potential errors? n M What level of accuracy do you expect? Q C t i i 0 i i Ideal Tracer same properties as fluid viscosity temperature density non reactive additional properties low background concentrations easily measured cheap non toxic Real Tracers Tracer type salt distinguishing property conductivity Dyes color fluorescent dye radioactive ions fluorescence Dissolved gas Gas radioactive decay analytical instrument Conductivity meter Spectrophotometer Fluorometer Liquid scintillation counter Gas chromatograph examples NaCl methylene blue rhodamine WT C14 Sulfur hexafluoride Reactor Lab Tracer Sodium chloride measured with conductivity probe Red dye # 40 so we can see it Density problem: 1.012 g/cm3 Which reactors would be affected by density difference? How can we solve it? 0.378C glucose 998.215 density (g/L) Density Matching 1040 glucose 1030 density = 0.378C + 998.215 1020 1010 1000 0.6985C NaCl 998.29 990 0 0.378C glucose 0.6985C NaCl 20 40 60 80 100 C (g/L) C glucose 1.848C NaCl density (g/L) Sodium chloride 1025 1020 1015 density = 0.6985C + 998.29 1010 1005 1000 995 0 10 20 C (g/L) 30 Monitoring Conductivity Probe location pipe flow porous media column completely mixed flow reactor Data acquisition conductivity probe monitored by meter that sends data to computer computer will display a graph of conductivity vs. time output is a tab delimited text file containing sample times conductivity Porous Media Reactor C x , t What M A 4Dd t ( x Ut ) 2 4 Dd t e are x, A, and U for the porous media reactor? How could we get the dispersion coefficient? What part of our laboratory model doesn’t this equation describe? Data Manipulation What would happen if you collected data n for a week? ti Ci t t i 0n C t No i 0 clear approach, perhaps eliminate data after 99% of the mass is accounted for? No need to collect data after the effluent concentration is stable. i Conductivity as f(NaCl) the slope of the four point calibration curve and the baseline conductivity of each of the reactors to convert the conductivity data to NaCl concentration Conductivity (mS/cm) Use 1500 y = 2.1680x + 6.7393 2 R = 1.0000 1000 500 0 0 200 400 600 600 NaCl concentration (mg/L) “Plug Flow” Completely Mixed Porous Media