Chapter 9: Chemical Calculations
advertisement

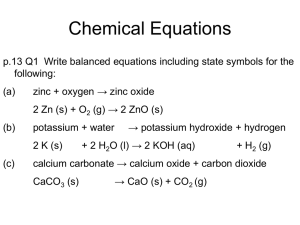
Chemical Calculations What does an equation tell us? A balanced chemical equation shows important facts about a reaction: Known as mole ratio a) The reactants b) The products c) The ratio of the amounts (in moles) of the reactants and the products d) The state of each reactants/products if indicated Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Stoichiometry It is the relationship between the amounts (measured in moles) of reactants and products involved in a chemical reaction. Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Stoichiometry Mg(s) + 2HCl(aq) 1 mol of magnesium 2 mol of hydrochloric acid MgCl2(aq) + H2(g) 1 mol of magnesium chloride 1 mol of hydrogen 1 mole of a substance is equal to its Mr in grams. Hence, 24 g of magnesium 73 g of hydrochloric acid 95 g of magnesium chloride Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. 2 g of hydrogen Example 1 Calculate the mass of the solid obtained when 16.8 g of sodium hydrogencarbonate is heated strongly until there is no further change. 2NaHCO3(s) Na2CO3(s) + CO2(g) + H2O(l) Mr of NaHCO3 = 23 + 1 + 12 + (3 × 16) = 84 Mr of Na2CO3 = (2 × 23) + 12 + (3 × 16) = 106 168 Number of moles of NaHCO3 used = 84 = 0.2 mol 2 mol of NaHCO3 produce 1 mol of Na2CO3. 1 Number of moles of Na2CO3 obtained = 0.2 × = 0.1 mol 2 Mass of solid obtained = number of moles of Na2CO3 × Mr of Na2CO3 = 0.1 × 106 = 10.6 g Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Example 2 63.0 dm3 of carbon monoxide, measured at r.t.p., was used to react with iron(II) oxide. What mass of iron was produced at the end of the reaction? [Ar of Fe = 56] Fe2O3(s) + 3CO(g) 2Fe(s) + 3CO2(g) Number of moles of carbon monoxide used = 63/24 = 2.625 mol 3 mol of carbon monoxide produce 2 mol of iron. Number of moles of iron produced in the reaction = 2.625 2/3 = 1.75 mol Mass of iron produced = number of moles of Fe × Ar of Fe = 1.75 × 56 = 98.0 g Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. How do we do calculations involving gases? In chapter 8, we learnt that 1 mole of any gas occupies 24 dm3 at room temperature and pressure. Hence, the volume of gas is proportional to the number of moles of the gas, and vice versa. Thus, we can change mole of gas to volume of gas. Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. How do we do calculations involving gases? N2(g) + 3H2(g) OR 2NH3(g) 24 dm3 of N2(g) + 72 dm3 H2(g) 48 dm3 NH3(g) Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Example 1 N2(g) + 3H2(g) 2NH3(g) a) Calculate the volume of ammonia gas produced when 20 cm3 of nitrogen gas was reacted with an excess of hydrogen gas. b) What is the volume of hydrogen required for this reaction? a) 1 mol of nitrogen produces 2 mol of ammonia, i.e. 1 volume of nitrogen produces 2 volumes of ammonia. Volume of ammonia produced = 2 × 20 = 40 cm3 b) Also, 1 volume of nitrogen reacts with 3 volumes of hydrogen. Volume of hydrogen required = 3 × 20 = 60 cm3 Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Example 2 At room temperature and pressure, 25 cm3 of butane (C4H10) exploded after it was mixed with an excess of oxygen. Calculate (a) the volume of carbon dioxide produced, and (b) the volume of oxygen required for the reaction. 2C4H10(g) + 13O2(g) 8CO2(g) + 10H2O(l) a) According to the equation, 2 volumes of butane produce 8 volumes of carbon dioxide. Volume of carbon dioxide produced = 8 × 25 = 100 cm3 2 b) 2 volumes of butane react with 13 volumes of oxygen. 13 Volume of oxygen required = × 25 = 162.5 cm3 2 Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Limiting Reactants Ideally, reactions should be carried out using exact quantities of reactants to reduce wastage. However, many reactions are carried out using an excess amount of one reactant. Why? This ensures that the more expensive reactant is completely used up. To do so, we make use of the idea of limiting reactants. Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Limiting Reactants H2(g) + Cl2(g) 2HCl(g) 1 mole of hydrogen reacts with to produce 2 moles of hydrogen chloride 1 mole of chlorine The reactants are in stoichiometric proportion. Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. All reactants are used up as they reacted in stoichiometric proportion. Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. reactants product The reactants that are not used up are called the excess reactants. The reactant that is completely used up in a reaction is known as the limiting reactant. In this case, it is chlorine. It is called the limiting reactant because it determines or limits the amount of products formed. Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. reactants product Here, hydrogen is the limiting reactant. Chlorine is the excess reactant. The amount of products formed in a reaction is always determined by the amount of the limiting reactant. Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Example 1 Zinc reacts with hydrochloric acid according to the equation Zn(s) + 2HCl(aq) ZnCl2(aq) + H2(g). If 0.05 mol of zinc was added to 0.075 mol of hydrochloric acid, a) identify the limiting reactant. b) calculate the amount (in moles) of the reactant that remained. a) 2 mol of HCl will react with 1 mol of Zn, hence 0.10 mol of HCl will react with 0.05 mol of Zn. 0.075 mol of HCl. Hence, amount of zinc used = (0.075/2) = 0.0375 mol of Zn. This is simple proportion Since 0.05 mol of Zn were used but only 0.0375 mol used, the zinc must be in excess and HCl is the limiting reactant. Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. (continued) Example 1 Zinc reacts with hydrochloric acid according to the equation Zn(s) + 2HCl(aq) ZnCl2(aq) + H2(g). If 0.05 mol of zinc was added to 0.075 mol of hydrochloric acid, a) identify the limiting reactant. (done) b) calculate the amount (in moles) of the reactant that remained. b) Amount of zinc which remained unreacted = 0.05 – 0.0375 = 0.0125 mol Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Example 2 Ethene, C2H4, burns in oxygen as shown in the equation. In an experiment, 10 cm3 of ethene was burnt in 50 cm3 of oxygen. a) Which gas was supplied in excess? Calculate the volume of the excess gas remaining at the end of the reaction. b) Calculate the volume of carbon dioxide produced. C2H4(g) + 3O2(g) 2CO2(g) + 2H2O(l) a) 1 volume of ethene reacts with 3 volumes of oxygen. Volume of oxygen used = 3 × 10 = 30 cm3 However, 50 cm3 of oxygen was used. Oxygen gas was in excess. Hence, volume of O2 remaining = initial volume – volume used = 50 – 30 = 20 cm3 Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Example 2 (continued) Ethene, C2H4, burns in oxygen as shown in the equation. In an experiment, 10 cm3 of ethene was burnt in 50 cm3 of oxygen. a) Which gas was supplied in excess? Calculate the volume of the excess gas remaining at the end of the reaction. (done) b) Calculate the volume of carbon dioxide produced. C2H4(g) + 3O2(g) 2CO2(g) + 2H2O(l) b) 1 volume of ethene produces 2 volumes of carbon dioxide. Volume of carbon dioxide produced = 2 × 10 = 20 cm3 Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Why is it important to identify the limiting reactant? Chemists choose the most expensive reactant to be the limiting reactant. This ensures all the expensive reactant is used up and not wasted. Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. The Concentration of a Solution Chemical reactions often involve solutions of substances in water. It is therefore important to know the concentration of a solution. Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. How do we determine the concentration of a solution? Concentration (g/dm3) = Mass of solute in grams Volume of solvent in dm3 1 dm3 is equivalent to 1000 cm3. Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Example 1 A solution of glucose contains 0.45 g of glucose in 75 cm3 of solution. What is the concentration of the glucose solution in g/dm3? Volume of glucose solution 75 = dm3 = 0.075 dm3 1000 Concentration of glucose solution mass = volume = 0.45 / 0.075 = 6.00 g/dm3 Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Molar Concentration The concentration of a solution can also be expressed in mol/dm3. When concentration is expressed in mol/dm3, it is called molar concentration. Concentration (mol/dm3) = Number of moles of solute Volume of solvent in dm3 or Concentration (mol/dm3) = Concentration (g/dm3) Mr 1 mol/dm3 means 1 mole of solute dissolved in 1 dm3 of solution. Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Example 1 A solution of sodium hydroxide was prepared by dissolving 3.5 g of sodium hydroxide in distilled water and making the volume up to 100 cm³. What is the concentration of the sodium hydroxide solution in mol/dm³? Mr of sodium hydroxide (NaOH) = 23 + 16 + 1 = 40 Number of moles of NaOH used = mass / Mr = 3.5 / 40 = 0.0875 mol Volume of NaOH = 100 cm3 = 100 / 1000 = 0.10 dm3 Concentration of NaOH solution in mol/dm3 = number of moles of NaOH / volume of solution (dm3) = 0.0875 / 0.10 = 0.875 mol/dm3 Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Example 2 The concentration of a bottle of hydrochloric acid is 73 g/dm3. How many moles of HCl are there in 250 cm3 of this acid? Mr of HCl = 1 + 35.5 = 36.5 Moles of HCl in 73 g = 73 / 36.5 = 2 mol 1000 cm3 of the HCl solution contains 2 mol of HCl. How many moles 250 cm3 will contain (250 / 1000) 2 = 0.5 mol of HCl Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. are there in 250 cm3 since there are 2 mol in 1000 cm3? Example 3 The concentration of a solution of HCl is 30 g/dm3. What is its concentration in mol/dm3? Concentration of HCl in mol/dm3 = (concentration in g/dm3) / Mr of HCl = 30 1 35.5 = 0.822 mol/dm3 Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Volumetric Analysis Many products that we use contain chemicals dissolved in water. To check the concentration of these substances, a chemist performs volumetric analysis. To do volumetric analysis, we use a method called titration. Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Titration - General Steps burette conical flask In titration, we determine the volume of a solution required to completely react with a known volume of another solution. By calculation, we can then determine the concentration of a solution. solution of unknown concentration Titration experiments that involve the use of an acid, e.g. HCl, and a base, e.g. NaOH, are called an acid-base titration. Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. How do we perform titration? The titrant is placed in the burette. The solution of unknown concentration is introduced into a conical flask using a pipette. One or two drops of indicator are then added to the conical flask. The titrant is allowed to react completely with a known volume (usually 25 cm3) of the solution of unknown concentration. Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. How do we perform titration? Titration is stopped at the end-point. The end-point is reached when the indicator permanently changes colour. The volume of titrant used is noted. The concentration of the unknown solution can then be calculated. Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Example 1 25 cm3 of the ammonia solution required 21.9 cm3 of 0.11 mol/dm3 sulphuric acid to achieve the end-point of titration. Calculate the concentration of ammonia, in g/dm3. H2SO4(aq) + 2NH3(aq) (NH4)2SO4(aq) Number of moles of sulphuric acid used in 25 cm3 = 0.11 × 21.9 / 1000 = 2.409 × 10–3 mol 1 mol of sulphuric acid reacts with 2 mol of ammonia solution. Number of moles of ammonia in 25 cm3 = 2 × 2.409 × 10–3 = 4.818 × 10–3 mol The ratio is 1:2. Hence the number of moles of ammonia is always twice in this reaction. Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Example 1 (continued) 25 cm3 of the ammonia solution required 21.9 cm3 of 0.11 mol/dm3 sulphuric acid to achieve the end-point of titration. Calculate the concentration of ammonia, in g/dm3. There are 4.818 10-3 mol in 25 cm3. There are 0.1927 mol in 1000 cm3. Conc = 0.1927 mol/dm3 Number of moles of ammonia in 1000 cm3 = 4.818 × 10–3 × 1000/25 = 0.1927 mol Mr of NH3 = 14 + (3 × 1) = 17 Concentration of ammonia in g/dm3 = 0.1927 × 17 = 3.28 g/dm3 Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Conc (in mol/dm3) = conc (in g/dm3) / Mr Example 2 16.6 g of a metal carbonate, M2CO3, was made up to 1000 cm3 of aqueous solution. 25 cm3 of this solution required 30 cm3 of 0.20 mol/dm3 HCl for complete reaction. a) Calculate the number of moles of HCl used in this reaction. b) Write the equation for the reaction between M2CO3 and HCl. c) Calculate the number of moles of M2CO3 present in i) 25 cm3 of solution. ii) 1 dm3 of solution. d) Calculate i) the relative atomic mass of M. ii) the relative formula mass (Mr) of M2CO3. e) Identify the metal M. Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Example 2 (continued) 16.6 g of a metal carbonate, M2CO3, was made up to 1000 cm3 of aqueous solution. 25 cm3 of this solution required 30 cm3 of 0.20 mol/dm3 HCl for complete reaction. a) Calculate the number of moles of HCl used in this reaction. b) Write the equation for the reaction between M2CO3 and HCl. a) Number of moles of HCl used = 0.2 × 30 = 0.006 mol 1000 b) The equation for the reaction is M2CO3(aq) + 2HCl(aq) 2MCl(aq) + H2O(l) + CO2(g) Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Example 2 (continued) 16.6 g of a metal carbonate, M2CO3, was made up to 1000 cm3 of aqueous solution. 25 cm3 of this solution required 30 cm3 of 0.20 mol/dm3 HCl for complete reaction. c) Calculate the number of moles of M2CO3 present in i) 25 cm3 of solution. ii) 1 dm3 of solution. M2CO3(aq) + 2HCl(aq) 2MCl(aq) + H2O(l) + CO2(g) c) i)1 mol of M2CO3 reacts with 2 mol of HCl. Since the number of moles of HCl used was 0.006, the number of moles of M2CO3 in 25 cm3 = 0.003 mol 1000 ii) There is 0.003 × 25 in 1000 cm3 (1 dm3) = 0.12 mol of M2CO3 in 1 dm3 of the solution. Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd. Example 2 (continued) d) Calculate i) the relative atomic mass of M. ii) the relative formula mass (Mr) of M2CO3. e) Identify the metal M. d) i) Let Ar of M be Q. The relative formula mass of M2CO3 = (2 × Q) + (1 × 12) + (3 × 16) = 2Q + 60 Number of moles of M2CO3 in 16.6 g = 16.62Q + 60 From (c)(ii), 0.12 = 16.62Q + 60 0.24Q + 7.2 = 16.6 Q = 39.2 ii) Relative formula mass = 2 × 39.2 + 60 = 138.4 e) The Ar of M is 39.2. M is potassium Copyright © 2006-2011 Marshall Cavendish International (Singapore) Pte. Ltd.