Organometallic Compounds: Reactions & Synthesis
advertisement

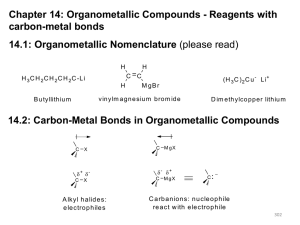
Organometallic Compounds Compounds that contain a metal-carbon bond (R-M): Examples of M include Li, Mg (Grignard reagents), Na, Cu, and Zn. Nucleophilic carbon: Reacts with electrophilic carbon and forms a new carbon-carbon bond. Nomenclature: Similar naming to substituted metals (alkyl metals or alkyl metal halides). Organomagnesium: Grignard reagent. CH3Li: Methyl lithium. CH3MgBr: Methyl MgBr. Learning Check Which (if any) of the following would not be classified as an organometallic substance? A) triethylaluminum B) ethylmagnesium bromide C) potassium tert-butoxide D) none of these (all are organometallic compounds) Organometallic Compounds Properties: High Reactivity: Organmometallics are usually kept in organic solvents due to their very high reactivity. Reacts especially with H2O, O2, etc. RMgX + H2O RH + MgOHX RMgX + O2 ROO- + MgOHX Organometallic Compounds Structure: Ionic Character: R-M = R- M+ NaR and KR are ionic. Electronegativities: Na: 0.9, K: 0.8, C: 2.5 CH3F LiR and MgR have a sigma bond between the carbon atom and the metal. These are very polar compounds. Electronegativities: Li: 1.0, Mg: 1.2 CH3Li Organometallic Compounds Reactivity: Bases act as nucleophiles. Basicity: Simple carbanions are strong bases. Carbon is not very electronegative compared to nitrogen or oxygen. RLi + HOR RH + Li+ -OR RMgX + HOR RH + Mg2+ -ORX- RLi or RMgX CANNOT be used in the presence of acidic hydrogens, such as –OH, -NH, or –SH. Learning Check Explain the pKa order of ethane, ethene and ethyne in the chart shown in your notes: Answer: % s character. Ethyne has an sp orbital. Ethene has an sp2 orbital. Ethane has an sp3 orbital. s character: (sp = 50%, sp2 = 33%, and sp3 = 25%). Increased s = closer to nucleus = more stabilization of electron pairs = more stable conjugate base formation. General Mechanisms 1. Nucleophilic Substitution: R2CuLi reaction with alkyl halides or tosylates. Nu C Nu C LG LG 2. Nucleophilic Addition: RLi or RMgX with aldehydes or ketones. O Nu Nu C O C H Nu C OH 3. Nucleophilic Acyl Substitution: RLi or RMgX with esters. O O Nu Nu C LG C LG O Nu C LG Then Nucleophilic addition Organolithium Reagents RX + 2 Li RLi + Li+X(X = I, Br, Cl) Reaction Type: Oxidation-Reduction. Alkyl halides react with lithium metal. Other Group I metals (Na, K) can be used instead of lithium. Solvents: Anhydrous diethyl ether, pentane, hexane. Alkyl group can be primary, secondary or tertiary. R can be alkyl, vinyl or aryl. Halide Reactivity: I > Br > Cl. Organolithium Reagents RX + 2 Li RLi + Li+X(X = I, Br, Cl) Limitations: Organolithium, RLi, is too basic for an SN1 or SN2 with alkyl halides or tosylates. This gives elimination reaction or other side reactions. Learning Check Which one of the following is not a suitable solvent for the reaction shown? CH3CH2CH2CH2Li + CH3CH=O CH3CH2CH2CH(OLi)CH3 A) hexane B) benzene C) ethanol D) diethyl ether Learning Check 1. What is the oxidation state change for the carbon in the reaction methyl iodide to methyl lithium? Reduction: -2 to -4. 2. What is the oxidation state change for the lithium in the reaction methyl iodide to methyl lithium? Oxidation: +1 to 0. 3. What is the oxidation state change for the iodide in the reaction methyl iodide to methyl lithium? -1 to -1: No change. Organomagnesium Reagents Et2O RX + Mg RMgX (X = I, Br, Cl) Reaction Type: Oxidation-Reduction. Alkyl halides react with magnesium metal. Solvents: Anhydrous diethyl ether, tetrahydrofuran (THF) Alkyl group can be primary, secondary or tertiary. R can be alkyl, vinyl or aryl. Halide Reactivity: I > Br > Cl. Organomagnesium Reagents Et2O RX + Mg RMgX (X = I, Br, Cl) Limitations: Organomagnesium, RMgX is too basic for SN1 or SN2 with alkyl halides or tosylates. In these situations, RMgX gives elimination reactions and other side reactions. Common Reactions with Carbonyl Groups: Aldehydes/Ketones: RLi and RMgX react with the carbonyl Organomagnesium Reagents Et2O RX + Mg RMgX (X = I, Br, Cl) Common Reactions with Carbonyl Groups: Aldehydes/Ketones: RLi and RMgX react with the carbonyl (C=O) to create alcohols. Addition to methanal (formaldehyde) 1° alcohol. Addition to other aldehydes 2° alcohol. Addition to ketones 3° alcohol. The acidic work-up intermediate metal alkoxide salt alcohol via a simple acid-base reaction. Organomagnesium Mechanism RLi or H O RMgX R C H H C H O H H RMgX R' O R C H R' C R' O H O RMgX C R' R R" KETONE Step 1: Nucleophilic C of organometallic reagent adds to electrophilic C in polar carbonyl group. Electrons move from C=O to electronegative O. R C OH H H 2 alcohol R' R' o ALDEHYDE RLi or OH C H 1o alcohol FORMALDEHYDE RLi or R C O H R C R" OH R" 3o alcohol Step 2: Work up step. Acid/Base reaction. Protonation of alkoxide oxygen creates alcohol from intermediate. Reactions usually in Et2O or THF followed by H3O+ workups Learning Check How many signals in the 13C-NMR spectrum would you expect for the product of the reaction of propylmagnesium bromide with formaldehyde (H2C=O) followed by H3O+? A) 2 B) 3 C) 4 D) 5 Esters Reaction Type: Nucleophilic Acyl Substitution, then nucleophilic addition. Requires 2 equivalents of organolithium or Grignard reagents. Product: 2° or3° alcohols. The alcohol contains 2 identical alkyl groups via a ketone intermediate which reacts with the second equivalent of the organometallic. Since the ketone is more reactive than the ester, the reaction cannot stop at a ketone. Grignard Reactions R’ = Alkyl, vinyl, aryl. X = Cl, Br, I R’CH2OH R’X H2CO Mg/Ether R’2CHOH HCO2Et R’CHROH RCHO R’R2COH RCOR RCN R’MgX (EtO)2CO R’COR H2O or acidic H O R’3COH RCO2Et R’2RCOH CO2 R’CO2H R’H R’CH2CH2OH Learning Check What is the product of the reaction of ethylmagnesium bromide (CH3CH2MgBr) with butanal (CH3CH2CH2CH=O) followed by dilute acid? A) 2-hexanol B) 1-butanol C) 3-hexanol D) 3-pentanol Organocopper Reagents Et2O 2 RLi + CuX R2CuLi + Li+X(X = I, Br, Cl) Lithium Dialkylcuprates, R2CuLi Two organolithiums with a copper (I) halide. Solvents: Anhydrous diethyl ether, tetrahydrofuran (THF) The alkyl group is usually primary. Secondary and tertiary are prone to decomposition. R can be alkyl, vinyl or aryl. Halide Reactivity: I > Br > Cl. Organocopper Reagents Et2O 2 RLi + CuX R2CuLi + Li+X(X = I, Br, Cl) Limitations: Organocuprates, R2CuLi, are less reactive (do not react with aldehydes, ketones or esters) and can be reacted with alkyl halides or tosylates to give alkanes. Organocopper Reagents Alkane Synthesis Using R2CuLi: R2CuLi + R’-X R-R’ + RCu + LiX Reaction Type: Nucleophilic Substitution. Creation of new C-C bonds. 1 alkyl iodides are best, otherwise an elimination reaction can occur. The R’ group in the halide can be aryl or vinyl. The R group of the cuprate can be aryl or vinyl. Although the mechanism looks like a SN2 reaction, it is more complex and is not well understood. Learning Check What is the principal organic product of the reaction of trans-1-bromo-1-butene with lithium diethylcuprate? A) B) C) D) cis-3-hexene trans-3-hexene 3-ethyl-3-hexene trans-2-hexene Learning Check 1. How can you tell that the alkane synthesis using R2CuLi is not a simple SN2 reaction? Aryl and vinyl substances can not do SN1 or SN2 easily. Organozinc Reagents Et2O RX + Zn RZnX (X = I, Br, Cl) Reaction Type: Oxidation-Reduction RZnX is made in a similar fashion as RMgX RZnX is less reactive than RLi or RMgX with aldehydes and ketones. The most common application of organozinc reagents is in the Simmons-Smith Reaction. Organozinc Reagents Synthesis of Cyclopropanes using RZNX Known as the Simmons-Smith Reaction Et2O ICH2I + Zn I-CH2-ZnI Cu Et2O I-CH2-ZnI + =—R R + ZnI2 Iodomethyl zinc iodide is usually prepared using Zn and activated with Cu. Iodomethyl zinc iodide reacts with an alkene to give a cyclopropane. Organozinc Reagents Synthesis of Cyclopropanes using RZNX Reaction is stereospecific with respect to the alkene. Mechanism is concerted. Substituents that are trans in the alkene are trans in the cyclopropane. Substituents that are cis in the alkene are cis in the cyclopropane. CH3 CH3 I-CH2ZnI ZnI2 CH3 Et2 O CH3 I-CH2ZnI ZnI2 CH3 CH3 Et2 O CH3 CH3 Organozinc Reagents Mechanism of the Simmons-Smith Reaction + ZnI2 CH2 I CH2 ZnI Reaction is concerted. Both new C-C bonds are formed simultaneously. Nucleophilic C=C causes loss of iodide leaving group. Electrons from nucleophilic C-Zn bond form the other C-C bond. Acetylenic Reagents B:- (B:- = NaNH2) R—CΞC—H R—CΞC:- + B-H Or B:- (B:- = R’MgX) R—CΞC—H R—CΞC:- MgX + R’-H Reaction Type: Acid-Base. Reacts as a carbanion. 3 important groups of reactions where nucleophiles attack electrophilic C atoms. Product is new C-C bonds. Acetylenic Mechanism RC CLi or RC CMgX H O RC C H C H C H O H RC O RC C H C R' H 1o alcohol R' R' C O H RC CLi RC CMgX R' C RC C R" C OH R' O H RC C R" KETONE 3o alcohol Addition to methanal (formaldehyde) gives primary alcohol. Addition to other aldehydes gives secondary alcohols. C R" C=O in aldehydes/ketones alcohols. Addition to ketones gives tertiary alcohols. C o R' O or C OH H 2 alcohol H ALDEHYDE RC C H FORMALDEHYDE RC CLi or RC CMgX C OH