11Soilacidity09

Understanding pH
pH = - log (H
+
ion concentration)
?
Brady and Weil, 2002
Optimum pH ranges have been identified for many crops
Native species also have pH preferences http://asecular.com/forests/graphics.jpg
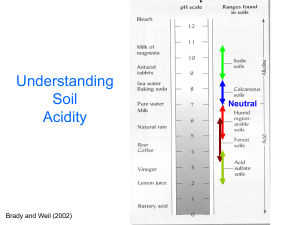
Understanding
Soil pH
Neutral
Brady and Weil (2002)
Do plants actually care about soil pH?
The acid infertility complex
Problems related to nutrient availability and metal toxicity in acid soils
For most soils and crops,
Nutrient total nutrient availability is availability optimized between varies with pH pH 5.5 and 7.
Molybdenum is more available at high pHs.
most http://www.farmtested.com/research_pp.html
Understanding aluminum toxicity
Toxic forms
Aluminum toxicity is above pH 5.5
http://www2.ctahr.hawaii.edu/tpss/research_extension/rxsoil/alroot.gif
Crop varieties differ in their sensitivity to Al toxicity
Brady and Weil, 2002
Reserve vs. active acidity
Reserve acidity
Soil pH is primarily a measure of active acidity
Active acidity
Brady and Weil, 2002
Understanding reserve acidity
Small pH change
BIG pH change
Reserve acidity
High CEC soil
Active acidity
Reserve acidity
Active acidity
Low CEC soil
Each charge depicted on this diagram represents 1 centimol of charge per kg of soil
SO
4
-2
+
+
Humus
Clay
-
-
-
-
K +
-
-
Ca +2
Mg +2
H +
What is the CEC of this soil ?
-
-
-
-
-
Al +3
K +
Ca +2
H
2
0
H
2
0 soil solution
H
2
0
H
2
0
H
H
2
0
2
0
+ 3H
2
O Al(OH)
3
+ 3H +
What is the “base” saturation ?
Each charge depicted on this diagram represents 1 centimol of charge per kg of soil
SO
4
-2
+
+
Humus
Clay
-
-
-
-
K +
-
-
Ca +2
Mg +2
H +
Many people refer to non-acid cations as base cations
-
-
-
-
-
K +
Al +3
Ca +2
H
2
0
H
2
0 soil solution
H
2
0
H
2
0
H
H
2
0
2
0
+ 3H
2
O ↔ Al(OH)
3
+ 3H +
What is the “base” saturation of this soil?
100 80 60 40 20 0
Acid Saturation, %
Soil acidity increases when H + producing processes exceed H + consuming processes.
Many processes add H + ions to soils
1) Carbonic acid forms when carbon dioxide dissolves in water.
H + ions are released when carbonic acid dissociates:
H
2
CO
3
-> H + + HCO
3
-
2) Organic acids form during the decomposition of organic matter.
H + ions are released when these organic acids dissociate.
3) Sulfuric and nitric acids form during the oxidation of reduced forms of N and S (e.g., NH
4
+ from fertilizer , elemental S ).
4) Sulfuric and nitric acids form when sulfur oxides and nitric oxides
(released into the atmosphere by automobile emissions, industry smoke stacks, volcanoes, forest fires) dissolve in precipitation.
H
2
SO4 and HNO
3 are strong acids and fully dissociate in water.
5) Roots release H + to balance internal charge when cation uptake exceeds anion uptake.
Many processes consume H + ions in soils
1) Weathering of most minerals (e.g., silicates, carbonates…)
2) Decomposition of organic anions
3) Reduction of oxidized forms of N, S and Fe.
4) Roots release OH or HCO
3
to balance internal charge when anion uptake exceeds cation uptake.
5) Inner sphere adsorption of anions (especially sulfate)
Sources of pH buffering
Carbonates
Lime
(CaCO
3
↓
) fountain of soil youth?
Young soil Old soil
Chadwick and Chorover ( 2001)
Acid inputs promote leaching of non-acid cations
Brady and Weil, 2002
S and N oxides cause acid precipitation
Brady and Weil, 2002
mineralization plant uptake
How much lime should be applied ?
Alfalfa field with dead strip where lime was not applied
Lime requirements should be guided by soil testing
Pocket pH meters can be very useful but require regular calibration !!!
Salt pH vs. water pH
Why do labs in arid regions use a salt solution?
Brady and Weil, 2002
Sources of variation in soil pH measurements
1. The nature and type of inorganic and organic constituents that contribute to soil acidity.
2. The soil to solution ratio used in measuring pH.
3. The salt content of the diluting solution used to achieve the desired soil to solution ratio.
4. The carbon dioxide content of the soil and solution.
5. Errors associated with standardization of the equipment used to measure pH.
The amount of lime needed to bring about a 1 unit change in pH varies widely between soils
“Illinois method” of determining lime requirement
How do you know which line to use ?
http://iah.aces.uiuc.edu/pdf/Agronomy_HB/11chapter.pdf
Choosing the right line
Line A: Dark colored silty clays and silty clay loams (CEC > 24)
Line B: Light and medium colored silty clays and silty clay loams, dark colored silts and clay loams (CEC 15-24)
Line C: Light and medium colored silt and clay loams, dark and medium colored loams, dark colored sandy loams (CEC 8-15)
Line D: Light colored loams, light and medium colored sandy loams and all sands (CEC < 8)
Line E: Mucks and peat (organic soils).
Light colored (< 2.5% OM)
Medium colored (2.5-4.5% OM)
Dark colored (4.5% OM)
Lime requirements determined using the “Illinois method” assume the following:
A. A 9-inch tillage depth .
If tillage is less than 9 inches, reduce the amount of limestone; if more than 9 inches, increase the lime rate proportionately. In no-till systems, use a 3-inch depth for calculations
Rates of lime should be
deep).
adjusted if any of these
B. Typical fineness of limestone .
Ten percent of the particles are
assumptions are not
mesh; 30 percent pass a 30-mesh and are held on 60-mesh; and 30 percent pass a 60-mesh.
accurate
C. A calcium carbonate equivalent (total neutralizing power) of 90 percent .
The rate of application may be adjusted according to the deviation from 90.
It takes time for lime to react in soil
Soil pH and lime requirement can vary widely within fields
Both past management and inherent soil properties affect soil pH and lime requirement
Insufficient lime is applied in IL to neutralize the acidity from N fertilizers http://iah.aces.uiuc.edu/pdf/Agronomy_HB/11chapter.pdf
Have you ever seen a stream look like this ?