Introduction
advertisement

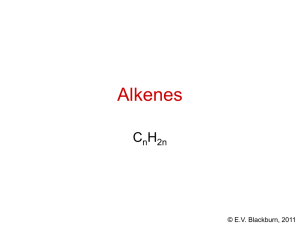
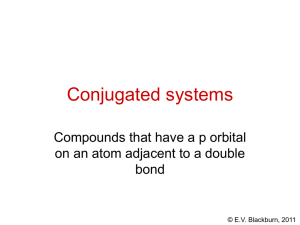
The chemistry of organic compounds © E.V. Blackburn, 2011 What is “organic chemistry” and why is it so important? • Organic chemistry is the study of the compounds of carbon. • Think about how organic compounds affect our daily life: • Our clothes – natural and synthetic fibers • Our medicines • Our food – carbohydrates, proteins, triglycerides • Oils, perfumes, paints, plastics, detergents, etc. © E.V. Blackburn, 2011 What is “organic chemistry” and why is it so important? O OH OH O O N H H N alizarin: the first naturally occurring dye to be synthesized (1868) indigo: used to dye blue jeans O CH 3CH 2OH ethanol: a fermentation product © E.V. Blackburn, 2011 “Vital force” theory Friedrich Wöhler (1828): NH 4+ NCO - H2N-C-NH 2 O ammonium cyanate urea A slight problem! The ammonium cyanate was synthesized from bones.......but the Kolbe synthesis of acetic acid in 1845 put the theory to rest! © E.V. Blackburn, 2011 Vitamin B12 The synthesis of vitamin B12 was finally completed in 1972 by Woodward and Eschenmoser after 10 years of work and the assistance of roughly one hundred graduate students. © E.V. Blackburn, 2011 “A production of amino acids under possible primitive earth conditions” S.L. Miller, Science, 117, 528 (1953) CH4 + NH 3 + H2O + H2 electric discharge amino acids including glycine and alanine © E.V. Blackburn, 2011 Proteins In order to give you an idea of the vastness of organic chemistry, we will look at proteins, our final topic of study in CHEM 263! E. coli contains ~5,000 different chemical compounds of which 3,000 are proteins. Man contains ~2,000,000 different proteins. Biologists believe that there are in excess 10,000,000 of proteins which take part in the process of life! http://www.wisegeek.com/how-many-proteinsexist.htm © E.V. Blackburn, 2011 Angiotensin II Angiotensin II is a blood pressure regulating hormone. It contains 8 amino acid residues. It is possible to arrange these in 40,320 different ways only one of which corresponds to the hormone! Its structure is actually: Asp-Arg-Val-Tyr-Ile-His-Pro-Phe. © E.V. Blackburn, 2011 Organic chemistry A logical subject based on fundamental principles: Can we predict the reactivity of a group of atoms based on what we’ve learned in school chemistry courses? The answer is YES! You know a great deal about acetone so let us look at it. © E.V. Blackburn, 2011 Acetone Here is acetone! But…where are the carbons and hydrogens? © E.V. Blackburn, 2011 Acetone Here is acetone! But…where are the carbons? © E.V. Blackburn, 2011 Acetone But…where are the hydrogens? © E.V. Blackburn, 2011 H3C C O H3C C C H H H C H C C H H H H C C C C H 1o CH3CH2CH3 H o 2 H H H H H H H H H H H H H H H 3o H © E.V. Blackburn, 2011 The chemistry of acetone © E.V. Blackburn, 2011 Acetone - a base! O H H O+ H H O+ + H2O Remember the curved-arrow convention. The tail points to the electron source and the head to the electron destination. © E.V. Blackburn, 2011 Acetone - a Lewis acid! O d+ NH 3 O C NH 3 © E.V. Blackburn, 2011 Models of chemical bonding These models are based on the premise that atoms react to produce the electronic configuration of a noble gas. © E.V. Blackburn, 2011 Models of chemical bonding: the ionic bond Consider the Li - F bond. Li F 1s2 2s1 1s2 2s2 2p5 - gives He configuration on loss of e- gives Ne configuration on gain of eLi - e- Li+ F + e- F- “Ions” are the structural units of ionic compounds and have strong electrostatic forces holding them together. © E.V. Blackburn, 2011 Models of chemical bonding: the covalent bond When atoms of similar electronegativities are bonded, complete electron transfer cannot take place. The noble gas configurations are attained by sharing electrons: Electronegativity - the ability of an atom to attract electrons. In the covalent bond, the atoms share electrons. The structural unit is the “molecule.” H H C H H © E.V. Blackburn, 2011 Lewis structures 1. Find the total number of valence electrons of all of the atoms. 2. Use pairs of electrons to form bonds between all bonded pairs of atoms. 3. Distribute the remaining electrons to give each hydrogen a duet and atoms of the second period an octet. e.g. CH4 e.g. C2H4 H H C H H H H C C H H © E.V. Blackburn, 2011 Lewis structures (CH3)2CHCH2OH 1. Find the total number of valence electrons of all of the atoms. 4 x 4 + 10 x 1 + 6 = 32 2. Use pairs of electrons to form bonds between all bonded pairs of atoms. 28 electrons © E.V. Blackburn, 2011 Lewis structures (CH3)2CHCH2OH 3. Distribute the remaining electrons to give each hydrogen a duet and atoms of the second period an octet. © E.V. Blackburn, 2011 Formal charge It is often necessary to include a formal charge when we draw a structure. Thus in drawing the hydronium ion we need to know where the charge is located. formal = valence - unshared electrons - 0.5 x shared electrons charge electrons in bonded atom in bonded atom in free atom H3O+ - for oxygen, formal charge = 6 - 2 - 0.5 x 6 = +1 What is the formal charge on nitrogen in the ammonium ion? © E.V. Blackburn, 2011 O O 1 O 2 3 formal = valence - unshared electrons - 0.5 x shared electrons charge electrons in bonded atom in bonded atom in free atom Formal charge for atom 1 = 6 - 6 (non-bonded e-) - 0.5 x 2 = -1 Formal charge for atom 2 = 6 - 2 - 0.5 x 6 = +1 Formal charge for atom 3 = 6 - 4 - 0.5 x 4 = 0 © E.V. Blackburn, 2011 Resonance Consider the carbonate ion, CO32-. We can draw three equivalent structures: - O - O - O O- - O O O O O- In reality the ion is perfectly symmetric. All C-O bond lengths are identical and the negative charge is delocalized over the three oxygens. The structure is a hybrid of these three contributing structures. © E.V. Blackburn, 2011 The theory of resonance • Whenever a molecule can be represented by 2 or more structures which differ only in the arrangement of their electrons, there may be resonance: - O - O - O O- - O O O O O- • The molecule is a hybrid of all the contributing structures and cannot be adequately represented by any one of these structures. © E.V. Blackburn, 2011 The theory of resonance and O H3C and O- O H3C O- OH and H3C H3C O O+ OH ??? © E.V. Blackburn, 2011 The theory of resonance • Resonance is important when these structures are of about the same stability. For example, O H3C However: H3C OO OH Oand H3C O OH3C + OH • The hybrid is more stable than any of the contributing structures. This increase in stability is called the resonance energy. © E.V. Blackburn, 2011 Benzene © E.V. Blackburn, 2011 Benzene © E.V. Blackburn, 2011 Benzene © E.V. Blackburn, 2011 Benzene © E.V. Blackburn, 2011 How to draw resonance structures CH2=CH-CH 2 CH2=CH-O-CH 3 CH2-CH=CH 2 + CH2-CH=O-CH 3 © E.V. Blackburn, 2011 How to draw resonance structures CH2=CH-CH 2 CH2-CH=CH 2 © E.V. Blackburn, 2011 How to draw resonance structures - 1,3-dienes + - - + H H H H H FC = 4 - 0 - 0.5x6 H = +1 FC = 4 - 2 - 0.5x6 = -1 © E.V. Blackburn, 2011 How to draw resonance structures - benzene © E.V. Blackburn, 2011 How to draw resonance structures + CH2=CH-CH 2 + CH2-CH=CH 2 © E.V. Blackburn, 2011 How to draw resonance structures Draw all the resonance structures of: H H - H H + H H H H H H H . H H © E.V. Blackburn, 2011 Quantum mechanics In order to understand covalent bonding, we must start by studying the electronic structure of individual atoms. Schrödinger calculated mathematical expressions which describe the motion of electrons. These “wave equations” give the energy levels available to electrons as well as the relative probability of finding an electron associated with a given energy level at any point in space. Orbitals are 3-dimensional representations of these probabilities. © E.V. Blackburn, 2011 Atomic orbitals Atomic orbitals are described by three quantum numbers: The most important is the principal quantum number, n. It governs the energy of an orbital. It can be equal to any positive integer except for zero. The second is the angular momentum quantum number, l, whose value depends on n: l = 0, 1, 2, …. n-1. It determines the shape of the orbital. The third is the magnetic quantum number, ml, which governs the orientation of the orbital relative to the three axes. ml = -l, -l + 1 … 0 … l - 1, l. © E.V. Blackburn, 2011 Atomic orbitals Orbitals are classified (named) according to their values of n and l using a number and a letter. The number represents the value of n and the letter represents the value of l. Electrons in an orbital having l = 0 are called s electrons. Electrons in an orbital having l = 1 are called p electrons. Electrons in an orbital having l = 2 are called d electrons. © E.V. Blackburn, 2011 Atomic orbitals n = 1, l = 0, ml = 0 one 1s orbital n = 2, l = 0, ml = 0 one 2s orbital n = 2, l = 1, ml = -1, 0, or +1 three 2p orbitals! © E.V. Blackburn, 2011 Atomic orbitals y z x y z 1s y y x x 2px z 2py z x 2pz © E.V. Blackburn, 2011 1s 2s 2p © E.V. Blackburn, 2011 Phase signs When the value of a wave equation is calculated for a particular point in space relative to the nucleus, the result may be a positive number, a negative number, or zero. y z node Y (+) x Y (-) 2py © E.V. Blackburn, 2011 Electron configurations The aufbau principle: Orbitals of lowest energy are filled first. The Pauli exclusion principle: Orbitals can accommodate a maximum of two electrons but only if they are of opposite spin. Hund’s rule: One electron is placed in each degenerate orbital before adding a second electron to an orbital. The electronic configuration of carbon is therefore 1s2 2s2 2p1 2p1 © E.V. Blackburn, 2011 Linear combination of atomic orbitals + Representation of the formation of the H-H bond by the sharing of electrons by the two hydrogens and overlap of their singly occupied atomic orbitals. © E.V. Blackburn, 2011 Molecular orbitals repulsion no interaction 436 kJ maximum stability 0.74 The H-H bond © E.V. Blackburn, 2011 s bond formation © E.V. Blackburn, 2011 Antibonding molecular orbitals When two atomic orbitals combine, they form two molecular orbitals. We have met the bonding molecular orbital. Here the atomic orbitals combine by addition. Thus orbitals of the same phase sign overlap. + The antibonding molecular orbital is formed by interaction of orbitals of opposite phase sign: + node © E.V. Blackburn, 2011 Energy diagram for the hydrogen molecule © E.V. Blackburn, 2011 The structure of methane Ground state electronic configuration of carbon: 1s 2s 2p H C H © E.V. Blackburn, 2011 The structure of methane Promote an electron to give four half filled atomic orbitals: 1s 2s 2p HH C H H © E.V. Blackburn, 2011 Hybrid atomic orbitals Orbital hybridization is a mathematical approach that involves the combination of individual orbital wave functions to obtain wave functions for new orbitals. These orbitals have, in varying proportions, the properties of the original orbitals taken separately. What does this mean for methane? © E.V. Blackburn, 2011 sp3 hybrid orbitals 1s 2s 2p The four orbitals are mixed (hybridized) to give four new sp3 hybrid orbitals which are oriented at angles of 109.5o and are more directional in character: © E.V. Blackburn, 2011 © E.V. Blackburn, 2011 Ethane - C2H6 H H C H H C H H H s H H C C H H s sp3 H © E.V. Blackburn, 2011 sp2 hybrid orbitals The electronic configuration of boron is 1s2 2s2 2p1. What is the structure of BF3? 1s 2s F F B 2p F o 120 © E.V. Blackburn, 2011 Structure - ethylene - C2H4 s H H 120o sp2 H H 120o H H H H © E.V. Blackburn, 2011 sp hybrid orbitals sp hybridization leads to a linear structure. BeH2 is an example of such a molecule. The 2s orbital and one of the 2p orbitals are hybridized. o 180 H Be H © E.V. Blackburn, 2011 Acetylene - C2H2 o 180 H C C H s sp H C C H © E.V. Blackburn, 2011 NH3 Ammonia is pyramidal and its bond angles (H-N-H) are 107o. .. N H H H © E.V. Blackburn, 2011 H2O Oxygen has 8 valence electrons in the water molecule: .. H O : H Its bond angle is 104.5. © E.V. Blackburn, 2011 Hybrid orbitals and carbon Using sp, sp2 and sp3 hybrid orbitals of carbon, we can construct the carbon chains and rings of organic compounds. The compound below is hystrionicotoxin, a non-protein based toxin that has been isolated from the skin of the “Poison Dart Frog”. The Golden Poison Dart frog may have enough toxin to kill 10 adult humans. Identify the hybrid orbitals to form the C-C, C-H, C-O and O-H bonds in this compound. Predict bond angles. OH N H © E.V. Blackburn, 2011 Functional groups – structural families Organic compounds are divided into “families” based on the groupings of atoms within the molecule. Lets now look at the “families” that we will encounter in CHEM 261/263. © E.V. Blackburn, 2011 Hydrocarbons These are compounds containing only carbon and hydrogen atoms. This “clan” is divided into smaller “family” units: • alkanes • alkenes • alkynes • aromatic compounds © E.V. Blackburn, 2011 Alkanes Alkanes do not have multiple bonds between carbon atoms. They are therefore “saturated” compounds. CH3CH2CH2CH3 butane © E.V. Blackburn, 2011 Alkenes Alkenes have a carbon – carbon double bond. They are therefore said to be “unsaturated” compounds. -pinene a component of turpentine © E.V. Blackburn, 2011 Alkynes Alkynes have a carbon – carbon triple bond and are therefore also “unsaturated”. O Capillin an antifungal agent © E.V. Blackburn, 2011 Benzene – a representative aromatic hydrocarbon We will study this group of unstaurated cyclic hydrocarbons in CHEM 263. Kekulé structures of benzene The six electrons are said to be “delocalized” over the six carbons of the benzene ring. © E.V. Blackburn, 2011 Alkyl groups An alkyl group is the structure obtained when a hydrogen atom is removed from an alkane. These groups are named by replacing the -ane suffix of the corresponding alkane by -yl, hence “alkyl”. • CH3- methyl (Me-) • CH3CH2- ethyl (Et-) • CH3CH2CH2- propyl (Pr-) “R” is used as a general symbol to represent any alkyl group. © E.V. Blackburn, 2011 Phenyl and benzyl groups CH 2 phenyl Ar- benzyl Ar-CH2- “Ar” is used as a general symbol to represent any aromatic ring system. © E.V. Blackburn, 2011 Functional groups A group of atoms and their associated bonds that has about the same chemical reactivity whenever it occurs in different compounds. © E.V. Blackburn, 2011 Alkyl halides R-X alkyl halide R = alkyl group (substituted or unsubstituted) H R R X R X H H 1o 2o R R X R 3o © E.V. Blackburn, 2011 carbon classification • a “primary” carbon is bonded to one other carbon • a “secondary” carbon is bonded to two carbon atoms • a “tertiary” carbon is bonded to three carbon atoms © E.V. Blackburn, 2011 Alcohols and phenols OH R OH alcohols phenols © E.V. Blackburn, 2011 O R O R ethers R H O O R R ketones R O R aldehydes carboxylic H acids O O O R esters R X acid halides R = alkyl or aryl © E.V. Blackburn, 2011 O O R R NH 2 amide O anhydride R O R C N nitrile R NH2 amine © E.V. Blackburn, 2011 The “hottest” compound This is probably capsaicin, the spicy constituent of peppers (capsicum annuum), cayenne pepper, chili peppers and other capsicum species. O N H HO OCH3 Identify the functional groups. © E.V. Blackburn, 2011 Bond Properties © E.V. Blackburn, 2011 Bond dissociation energies Energy is released when bonds are formed. Energy is absorbed when bonds are broken. This energy is called the bond dissociation energy, D. © E.V. Blackburn, 2011 Modes of bond breaking Homolysis A B A + B free radicals Heterolysis A B A + B ions © E.V. Blackburn, 2011 Breaking of bonds to carbon C Z + :Z- + carbocation C Z C:- + Z+ carbanion +Z C Z radical © E.V. Blackburn, 2011 Bond dissociation energies H for bond homolysis at 25C Bond broken H-H D-D F-F Cl - Cl Br - Br I-I H-F H - Cl H - Br H-I H3C - H H3C - F H3C - Cl H3C - Br H (kJ/mol) 436 444 159 243 193 151 570 432 366 298 438 452 351 293 Bond broken (CH3)2CH - H (CH3)2CH - F (CH3)2CH - Cl (CH3)2CH - Br (CH3)2CH - I (CH3)2CH - OH (CH3)2CH - OCH3 (CH3)2CHCH2 - H (CH3)3C - H (CH3)3C - Cl (CH3)3C - Br (CH3)3C - I (CH3)3C - OH (CH3)3C - OCH3 H (kJ/mol) 401 439 339 274 222 385 337 410 390 330 263 209 379 326 © E.V. Blackburn, 2011 Bond dissociation energies Bond broken H3C - I H3C - OH H3C - OCH3 CH3CH2 - H CH3CH2 - F CH3CH2 - Cl CH3CH2 - Br CH3CH2 - I CH3CH2 - OH CH3CH2 - OCH3 CH3CH2CH2 - H CH3CH2CH2 - F CH3CH2CH2 - Cl CH3CH2CH2 - Br CH3CH2CH2 - I CH3CH2CH2 - OH CH3CH2CH2 - OCH3 H (kJ/mol) 234 380 335 420 444 338 285 222 380 335 410 444 338 285 222 380 335 Bond broken C6H5CH2 - H CH2=CHCH2 - H CH2=CH - H C6H5 - H HCC - H H3C - CH3 CH3CH2 - CH3 CH3CH2CH2 - CH3 CH3CH2 - CH2CH3 (CH3)2CH - CH3 (CH3)3C - CH3 HO - H HOO - H HO - OH CH3CH2O - OCH3 CH3CH2O - H H (kJ/mol) 368 361 444 464 552 376 355 355 343 351 339 498 377 213 184 436 © E.V. Blackburn, 2011 H - CH3 + Cl - Cl Cl - CH3 + H - Cl 438 kJ 351 kJ 243 kJ 681 kJ 432 kJ 783 kJ H = + 438 + 243 - 351 - 432 = -102 kJ © E.V. Blackburn, 2011 Polar bonds and polar molecules A polar bond is a covalent bond between two atoms of differing electronegativity. d+ dH F d+ dO H H d+ © E.V. Blackburn, 2011 Dipole moments A polar molecule is one in which the centres of positive and of negative charge do not coincide. This uneven electron distribution is measured by a quantity called the dipole moment, , measured in units of debye. The direction of bond and molecule dipoles are often indicated using an arrow: positive end H F = 1.75D © E.V. Blackburn, 2011 Dipole moments Cl Cl Cl Cl Cl =0 H H H = 1.86D © E.V. Blackburn, 2011 Physical properties Physical properties such as melting point, boiling point and solubility are dependant on bond polarity and bond type (ionic or covalent). © E.V. Blackburn, 2011 Melting point The temperature at which the thermal energy of the structural units is great enough to overcome the intracrystalline forces that hold them together. © E.V. Blackburn, 2011 Melting points v structural unit ionic compounds High mp. Why? NaCl has a mp = 801C. Each sodium ion is surrounded by six chloride ions.....the crystal is very strong and the structure rigid. covalent compounds Lower mp. Why? The structural unit is the molecule. What are the intermolecular forces of attraction? © E.V. Blackburn, 2011 Dipole-dipole attractions The attraction of the positive ends of polar molecules by the negative ends of other polar molecules. Polar compounds therefore have higher mp and bp than non-polar compounds of comparable molecular mass. © E.V. Blackburn, 2011 Hydrogen bonding Hydrogen bonding is an example of a dipole - dipole interaction in which a hydrogen atom acts as a “bridge” between two very electronegative atoms. This attractive force is ~20 kJ/mol. Ethanol (CH3CH2OH) and dimethyl ether (CH3OCH3) have the same molecular formula. However ethanol has a boiling point of 78.3oC whereas that of the ether is -24.8oC. © E.V. Blackburn, 2011 van der Waals forces Methane has no net dipole moment. However when two molecules come in close contact, their electron clouds repel one another. The result is an induced dipole, albeit a small one. The electron deficient part of one molecule attracts the electron rich part of another. The dipoles continually change but the net result is an attraction between the molecules. © E.V. Blackburn, 2011 Criteria of purity All scientists observe systems, make observations and draw conclusions from their results. However, we must know for certain exactly what is under scrutiny. Otherwise any results obtained are simply a worthless jumble of irreproducible facts. Even a sample taken from a labeled bottle is no assurance of purity! © E.V. Blackburn, 2011 The peroxide effect HCl CH3CH=CH 2 CH3CHClCH 3 CH3CH=CH 2 CH3CH=CH 2 CH3CH=CH 2 HI H2O/H+ HBr CH3CHICH 3 CH3CH(OH)CH 3 CH3CH2CH2Br © E.V. Blackburn, 2011 The peroxide effect HBr CH3CH=CH 2 CH3CHBrCH 3 absence of peroxides HBr CH3CH=CH 2 CH3CH2CH2Br peroxides © E.V. Blackburn, 2011 Melting point - used to indicate degree of purity and in identification of a solid - recrystallize to constant melting point - a sharp melting point range (<1C) between appearance of drops of liquid within the sample to the disappearance of the last trace of solid - mixed melting points. Salt is often placed on an icy sidewalk. Why? © E.V. Blackburn, 2011 Boiling points Pure liquids which distil without decomposition will have sharp, constant boiling points and will leave no residue on distillation to dryness. However, boiling points are very pressure dependent making comparisons unreliable. Some liquid mixtures pose problems – azeotropic mixtures. © E.V. Blackburn, 2011 Refractive index Using an Abbé refractometer, the index can be measured and compared with that of an authentic sample or with literature values. An excellent method of evaluating the purity of a liquid. © E.V. Blackburn, 2011 Thin layer chromatography How can chemists prove that a compound is pure or identical with an authentic sample using chromatography? We can’t! If more than one spot or peak is present, we know that the sample is impure. The presence of one spot or peak is an indication of purity (or identity) but it can never constitute a proof of purity. © E.V. Blackburn, 2011 Elemental analysis C H O 52.24% 13.05% 34.71% 100g contains 52.24g 13.05g 34.71g or 52.24mol 13.05mol 34.71mol 12.01 1.008 16.00 4.36mol 12.93mol 2.16mol C4.36H12.93O2.16 C2H6O Empirical formula © E.V. Blackburn, 2011 Mass spectrometry - used to determine molecular weights - used to determine molecular formulas - used in structure elucidation © E.V. Blackburn, 2011 Infrared spectroscopy - bonds vibrate with characteristic frequencies. - absorption of IR energy occurs only when there is a match between the wavelength of the radiation and the wavelength of the bond vibration. - the position of the IR absorption depends on the strength of the bond, the masses of the bonded atoms and the type of vibration observed © E.V. Blackburn, 2011 Bond vibrations stretching vibrations A< >B A > <B bending vibrations B A B C A C © E.V. Blackburn, 2011 The 5000 to 1250 cm-1 region Absorptions between 5000 and 1250cm-1 can usually be assigned to a specific bond and a specific vibrational mode. © E.V. Blackburn, 2011 The finger print region Absorptions between 1250 and 675cm-1 are associated with more complex vibrational and rotational modes and are often characteristic of the molecule as a whole. This region is therefore called the “finger print region” of the IR spectrum. © E.V. Blackburn, 2011 Compound type Frequency (cm-1) Intensity Vibration alcohols a) RCH2OH 3600 3400 1050 v s s O-H unassociated O-H associated C-O stretching b) R2CHOH 3600 3400 1150 v s s O-H unassociated O-H associated C-O stretching c) R3COH 3600 3400 1200 v s s O-H unassociated O-H associated C-O stretching 1690 - 1750 2720 - 2850 s m C=O stretching C-H stretching aldehydes © E.V. Blackburn, 2011 Compound type alkanes Frequency Intensity Vibration (cm-1) 2850 - 3000 s C-H stretching 1450 - 1470 s CH2 and CH3 bending 1370 - 1380 s CH2 and CH3 bending 720 - 725 m CH2 and CH3 bending © E.V. Blackburn, 2011 Compound type alkenes a) RCH=CH2 b) R2C=CH2 c) (Z)-RCH=CHR d) (E)-RCH=CHR e) R2C=CHR f) R2C=CR2 Frequency Intensity Vibration (cm-1) 3080 - 3140 m =C-H stretching 1800 - 1860 m overtone 1645 m C=C stretching 990 s C-H bending 910 s C-H bending 3080 - 3140 m =C-H stretching 1750 - 1800 m overtone 1650 m C=C stretching 890 s C-H bending 3020 w =C-H stretching 1660 w C=C stretching 675 - 725 m C-H bending 3020 w =C-H stretching 1675 vw C=C stretching 970 s C-H bending 3020 w =C-H stretching 1670 w C=C stretching 790 - 840 s C-H bending 1670 vw C=C stretching © E.V. Blackburn, 2011 Frequency (cm-1) Compound type alkynes Intensity Vibration b) RCCR 3300 2100 - 2140 600 - 700 2190 - 2260 s m s vw C-H stretching CC stretching C-H bending CC stretching a) fluorides b) chlorides c) bromides d) iodides 1000 - 1350 750 - 850 500 - 680 200 - 500 vs s s s C-F C-Cl C-Br C-I a) RCCH alkyl halides © E.V. Blackburn, 2011 Compound type Frequency (cm-1) Intensity Vibration amines 3300 - 3500 1180 - 1360 m N-H stretching C-N stretching benzene ring 3000 - 3100 m C-H stretching 1600, 1500 690 - 710, 730 - 770 735 - 770 v C=C stretching C-H bending, mono subst. C-H bending, osubst. C-H bending, msubst. C-H bending, psubst 690 - 710, 750-810 810 - 840 © E.V. Blackburn, 2011 Compound type carboxylic acids Frequency Intensity Vibration (cm-1) 2500 - 3000 s, b O-H stretching 1700 - 1725 s C=O stretching, aliphatic C=O stretching, aromatic, or unstaturated C-O stretching O-H bending 1680 - 1700 s 1250 1400, 920 b esters 1715 - 1740 1050 - 1300 s s C=O stretching C-O stretching, 2 bands ethers 1070 - 1150 s C-O stretching ketones 1690 - 1750 s C=O stretching nitriles 2210 - 2260 v CN stretching © E.V. Blackburn, 2011 C 7 H8 O © E.V. Blackburn, 2011 Examining a spectrum • ~3400 cm-1: O-H and N-H stretch • ~3100 cm-1: sp and sp2 C-H stretch • ~2900 cm-1: sp3 C-H stretch • ~2750 cm-1: aldehyde C-H stretch • ~2200 cm-1: C≡C and C≡N stretch • ~1700 cm-1: C=O stretch • ~1600 cm-1: C=C stretch of alkenes and arenes • ~1200 cm-1: C-O stretch (limited utility) © E.V. Blackburn, 2011 Degree of unsaturation Degree of unsaturation = (2NC - NX + NN – NH + 2)/2 NC = number of carbons NX = number of halogens NN = number of nitrogens NH = number of hydrogens NH 2 H Cl O © E.V. Blackburn, 2011 C7H6O2 © E.V. Blackburn, 2011 C 7 H5 N © E.V. Blackburn, 2011 C 7 H9 N © E.V. Blackburn, 2011