Chapter 4 – Carbon and the Molecular Diversity of Life
advertisement
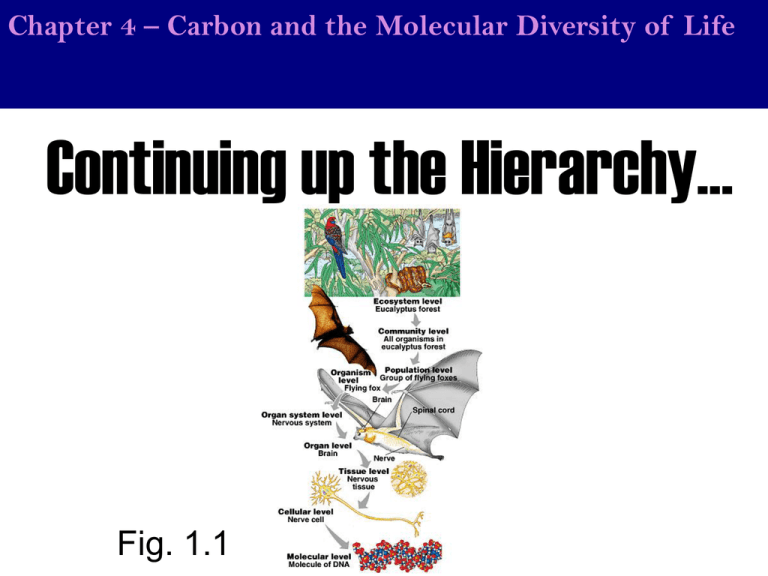
Chapter 4 – Carbon and the Molecular Diversity of Life Continuing up the Hierarchy… Fig. 1.1 Chapter 4 – Carbon and the Molecular Diversity of Life Each level of the hierarchy gets relatively less and less complex. Think about it. Protons, neutrons and electrons can combine in an infinite number of ways to make an infinite number of elements, but they don’t. There are only 115 known elements, and to make it simpler, only 88 occur naturally. To make it even more simpler, life could use these 88 elements, but it doesn’t. Life only uses 25 of them, and really only 11 in any considerable amount. Of these 11, 4 (CHON) make up 96% of the mass. Simpler and simpler. Now as you can imagine, these 25 elements could combine to form an infinite number of molecules making like extremely complex, but guess what…they don’t and this is what you will see in chapter 3. Chapter 4 – Carbon and the Molecular Diversity of Life The element that life is based on? Life is based on carbon. Why? Take the four major elements on life. Start with hydrogen and see how many different structures (molecules) you can make… You can make one – H2 – that will not work. Now try oxygen. You can make one – O2 – that won’t work. Perhaps nitrogen? Nope, one molecule again…– N2. Now try carbon. Carbon can make four bonds and will not quadruple bond to itself. Therefore you can make an infinite number of structures without a dead end; the structures of life. You can also attach all the other elements (H,N,O,S,P,etc…) to the carbons. Chapter 4 – Carbon and the Molecular Diversity of Life NEW AIM: Why Carbon? Why is carbon able to make four covalent bonds? Because it needs four valence electrons and will satisfy that need by sharing 4 electrons with other atoms. Chapter 4 – Carbon and the Molecular Diversity of Life AIM: Why Carbon? The study of carbon-based compounds? Organic Chemistry Organic chemistry is the field of chemistry that focuses on organic molecules. Organic molecules are molecules that contain BOTH Carbon and Hydrogen. They are produced NATURALLY SOLELY by organisms. We can make them “synthetically” in laboratories. Therefore, organic chemistry is the study of carbon/hydrogen based molecules, the molecules made and used by life. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? Is CO2 an organic molecule? No, because hydrogen is not present. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? Could organic compounds have been synthesized abiotically on the earth Earth as to later allow life to evolve? Chapter 4 – Carbon thelife Molecular of Life NEW AIM: Howand did beginDiversity on Earth? AIM: Why Carbon? Earth’s Beginning 4.6 Billion years ago 1. Earth coalesces from the stellar nebula (the great bombardment) Chapter 4 – Carbon thelife Molecular of Life NEW AIM: Howand did beginDiversity on Earth? AIM: Why Carbon? 2. Cooling Down Crust begins to solidify. (no atmosphere yet, too hot.) Chapter 4 – Carbon thelife Molecular of Life NEW AIM: Howand did beginDiversity on Earth? AIM: Why Carbon? 3. Formation of the atmosphere -Gases belched out from within the Earth punching holes in the crust (volcanoes; vents) Early Atmosphere: - Carbon monoxide (CO) - Carbon Dioxide (CO2) -Nitrogen (N2) - Water H2O - Methane (CH4) - Ammonia (NH3) - Hydrogen (H2) Atmosphere, but no oceans, still way too hot to have liquid water. Chapter 4 – Carbon thelife Molecular of Life NEW AIM: Howand did beginDiversity on Earth? AIM: Why Carbon? 4. Formation of the Oceans -Earth continues to cool… - water begins to condense - Torrential rain - Lightning - The oceans form Chapter 4 – Carbon and begin the Molecular Diversity of Life AIM: How did life on Earth? AIM: Why Carbon? So life appeared somewhere between the end of the great bombardment (4Bya) and the oldest known fossil (3.5Bya). Conclusion (How long did it take for life to develop?): <500 million years for life to appear!! Fig. 16.1C Chapter 4 – Carbon and begin the Molecular Diversity of Life AIM: How did life on Earth? AIM: Why Carbon? So how did life begin? What is required for there to be life as we know it? ORGANIC MOLECULES (monomers) Chapter 4 – Carbon and begin the Molecular Diversity of Life AIM: How did life on Earth? AIM: Why Carbon? Haldane Oparin 1920s - Oparin and Haldane first proposed that the Early conditions on Earth were sufficient to generate organic molecules. Chapter 4 – Carbon and begin the Molecular Diversity of Life AIM: How did life on Earth? AIM: Why Carbon? Haldane Oparin How would you test this hypothesis? Chapter 4 – Carbon and begin the Molecular Diversity of Life AIM: How did life on Earth? AIM: Why Carbon? 1953 - Stanley Miller - 23 year old grad student in the laboratory of Harry Urey at the University of Chicago Chapter 4 – Carbon and begin the Molecular Diversity of Life AIM: How did life on Earth? AIM: Why Carbon? Miller-Urey Experiment = early Earth simulation After one week: Found organic compounds - amino acids (abundant) Since then: Amino acids Sugars Lipids Fig. 16.3B Chapter 4 – Carbon and begin the Molecular Diversity of Life AIM: How did life on Earth? AIM: Why Carbon? Conclusion: Conditions on early Earth may have been sufficient to produce the organic molecules of life. Does that mean you have life? No, just organic molecules. Such experiments ruled out the idea of vitalism… The belief that a “life force” outside the laws of physics and chemistry was required to make organic molecules. Basically, organic molecules could only be made by living organisms. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? The simplest organic molecule and three-dimensionality The simplest organic molecule, methane (CH4). Notice how molecules are 3-Dimensional. When carbon attaches to four other atoms, a tetrahedral shape (three-sided pyramid with the carbon atom at the center) will be formed as the electrons in the bonds repel each other. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? Figure 4.3. The shapes of simple organic molecules. tetrahedral planar When 2 carbons are joined by a double bond as in (c), all bonds attached to these carbons are in the same plane (planar). Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? Drawing three-dimensionally: Make sure you can draw molecules this way Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? Drawing skeletal formula: = Skeletal formula Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? Examples Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? Hydrocarbons The organic molecules on the right as well as methane are all hydrocarbons. A HYDROCARBON is any molecule made of ONLY hydrogen and carbon. Carbon skeleton The chains, branches and/or rings of carbon atoms that form the basis of the structure of an organic molecule. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? The molecular formula does not necessarily tell you the structural formula…explain. C4H10 Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? ISOMERS C4H10 C4H8 1. Structural (constitutional) isomers Not to be confused with isotopes, structural isomers are molecules with the same molecular formula, but there atoms are connected differently (Different connectivity) resulting in different structural formula. Structure determines function and therefore structural isomers function or behave differently. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? ISOMERS Cis Trans X represents an atom or group of atoms attached to the double-bonded carbon, but of course is not hydrogen as hydrogen would result in these two molecules being identical. Example: Cis-2-butene Trans-2-butene 2. Geometric isomers Have the same connectivity, but differ in their spatial arrangement resulting in different 3D structures… Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? ISOMERS = Are these isomers? No. Recall that single bonds can rotate. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? ISOMERS ≠ What if we place a double bond between the carbons? Then yes, they are isomers since double/triple bonds cannot rotate. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? ISOMERS Cis Trans Cis (“on the same side” – latin) - results when the substituent groups (X) are on the same side. Trans (“across” – latin) - results when the substituent groups (X) are on opposite sides. 2. Geometric isomers Have the same connectivity, but differ in their spatial arrangement resulting in different 3D structures… Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? Cis ISOMERS Trans PRACTICE: trans cis cis trans 2. Geometric isomers Have the same connectivity, but differ in their spatial arrangement resulting in different 3D structures… Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? ISOMERS Trans-oleic acid (a trans fat) PRACTICE: cis-oleic acid 2. Geometric isomers Have the same connectivity, but differ in their spatial arrangement resulting in different 3D structures… Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? ISOMERS Are these two molecules isomers? No. If you turn the one on the right so that the amino group (NH2) faces you, it will look identical to the one on the left. These two molecules are the same. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? ISOMERS What about now? You can try all you like. You will not be able to get these two molecules to overlap each other. They are mirror images like your hands. Try to overlay your hands… Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? ISOMERS mirror What about now? You can try all you like. You will not be able to get these two molecules to overlap each other. They are mirror images like your hands. Try to overlay your hands… Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? ISOMERS mirror 3. Enantiomers Molecules that are mirror images of each other (cannot be overlayed and therefore have different spatial arrangments). What property of these molecules causes this to happen you ask? Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? ISOMERS Asymmetric carbon 3. Enantiomers – the asymmetric carbon This can happen only when there is an asymmetric carbon = a carbon with four DIFFERENT groups attached to it. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? ISOMERS Identify the asymmetric carbon(s) in the molecule above. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? ISOMERS Asymmetric carbon D-isomer L-isomer 3. Enantiomers – L and D We designate such mirror image molecules as either L- or D- from the latin for left and right (levo and dextro). In biology only one form is the active form. For example, all amino acids are L-isomers, while all sugars are D-isomers. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? ISOMERS Used/Found in biological organisms Not used/found in biological organisms 3. Enantiomers – L and D We designate such mirror image molecules as either L- or D- from the latin for left and right (levo and dextro). In biology only one form is the active form. For example, all amino acids are L-isomers, while all sugars are D-isomers. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? Thalidomide, an extreme example of isomers…what type? R-thalidomide is an incredible antiemetic (inhibits nausea and vomiting). When would such a drug be used? After getting anesthesia, chemotherapy or any drug that causes nausea, but also for morning sickness when pregnant. Thousands of pregnant women took this drug (molecule) in the late 50’s early 60’s to treat morning sickness, but what scientists didn’t realize was when this molecule was made in the lab, a second molecule was inadvertently made… S-thalidomide, an enantiomer of R-thalidomide. S-thalidomide, unfortunately, is a teratogen (teros; greek for monster, -gen; creation of). A teratogen is a molecule that causes birth defects. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? Thalidomide, an extreme example of isomers behaving differently The birth defects caused by the teratogen Sthalidomide, which was inadvertently taken with Rthalidomide to treat morning sickness symptoms Conclusion Just because two molecules have the same molecular formula and may even have the same connectivity, if they can’t be overlaid on top of each other, they aren’t the same. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? Find the structural isomers Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: Why Carbon? Summary: Fig. 4.7 Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: can we make hydrocarbons reactive at biological temperatures? AIM:How Why Carbon? Look at this hydrocarbon. Predict how reactive these kinds of molecules will be at ROOM TEMPERATURE or BODY TEMPERATURE and how readily it will dissolve in water. Explain your rationale. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: cancan we make hydrocarbons reactivemore at biological temperatures? AIM:How How we make hydrocarbons reactive? How can we make hydrocarbons more reactive with each other and water friendly at the same time to make them useful building blocks of life ? Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life NEW AIM: canhydrocarbons we make hydrocarbons more reactive and AIM: How canHow we make reactive at biological temperatures? soluble? Make them “sticky” Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: wewe make hydrocarbons reactive biological temperatures? AIM:How Howcan can make hydrocarbons moreatreactive and soluble? By adding highly electronegative elements (O, N, S, etc…), we can give the molecules partial and full charges. Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those Functional group (polar or charged) Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: cancan we make hydrocarbons reactivemore at biological temperatures? AIM:How How we make hydrocarbons reactive? We will now review the six most common functional groups. You need to know (be able to draw/identify) all six. 1. The hydroxyl group -If you see a structural diagram of a molecule and hanging off one end is –OH, it implies that the oxygen and hydrogen are attached by a covalent bond. Another example would be something like –CH3 which means the three hydrogens are covalently bound to the carbon (there is no other possibility). - Obviously the oxygen is partially negative and the hydrogen is partially positive due to differences in electronegativity - Compounds that have a hydroxyl are typically called alcohols. The example shown is ethanol (drinking alcohol), but there are countless others from the familiar isopropanol to the less common tert-butanol. - Notice that the names of alcohols typically end in –ol. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: cancan we make hydrocarbons reactivemore at biological temperatures? AIM:How How we make hydrocarbons reactive? 2. Carbonyl Group Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: cancan we make hydrocarbons reactivemore at biological temperatures? AIM:How How we make hydrocarbons reactive? 2. Carbonyl Group - It is simply a carbon DOUBLE-BONDED to an oxygen where the oxygen is partial negative and the carbon partial positive - If the carbonyl is found at the end of a carbon skeleton, the resulting compound (molecule) is called an aldehyde. If at the end of the molecule, the carbon will always be attached to a hydrogen (hence the “hyde” part of aldehyde). Such compound names will usually end in –al like propanal (shown above). Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: cancan we make hydrocarbons reactivemore at biological temperatures? AIM:How How we make hydrocarbons reactive? 2. Carbonyl Group - If the carbonyl is found within the carbon skeleton (not at the end), the resulting compound (molecule) is called a ketone. The names of such compounds typically end in –one like acetone (shown above). - The carbon of the carbonyl will be attached to two other carbons making a letter “T”, which rhymes with “key”. This is how I once remembered what a ketone – ketone has the letter T in it and T rhymes with key. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: cancan we make hydrocarbons reactivemore at biological temperatures? AIM:How How we make hydrocarbons reactive? The next functional group can be formed by adding the hydroxyl group to the carbonyl group… + = Carbonyl Hydroxyl Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: cancan we make hydrocarbons reactivemore at biological temperatures? AIM:How How we make hydrocarbons reactive? 3. Carboxyl Group Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: cancan we make hydrocarbons reactivemore at biological temperatures? AIM:How How we make hydrocarbons reactive? 3. Carboxyl Group - A carboxyl group is always at the end of a carbon skeleton since it always has a hydrogen attached to the oxygen (you can’t add anymore carbons. Both oxygens pull the electrons from the hydrogen making the - How tightly do you think that hydrogen NUCLEUS is being held?? hydrogen nucleus (proton) fall off VERY easily Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: cancan we make hydrocarbons reactivemore at biological temperatures? AIM:How How we make hydrocarbons reactive? 3. Carboxyl Group - What do you call a molecule that will lose a proton (hydrogen ions) to the solution it is in? You call it an ACID - This is why compounds (molecules) with a carboxyl group are called carboxylic acids and are acids in general. When the proton falls off, the oxygen will become negative (it gets the hydrogen’s electron). Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: cancan we make hydrocarbons reactivemore at biological temperatures? AIM:How How we make hydrocarbons reactive? 4. Amino Group Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: cancan we make hydrocarbons reactivemore at biological temperatures? AIM:How How we make hydrocarbons reactive? Amino groups are always at the ends of carbon skeletons and compounds containing them are called amines. The hydrogens are obviously partial positive and the nitrogen partial negative in charge. Amino groups can act as a base picking up a proton: -NH2 + H+ -NH3+ Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: cancan we make hydrocarbons reactivemore at biological temperatures? AIM:How How we make hydrocarbons reactive? phosphate H2PO4 sulfhydryl -SH Above are two additional functional groups you need to add to memory not found in the chart in your book. The phosphate group and the sulfhydryl group. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: cancan we make hydrocarbons reactivemore at biological temperatures? AIM:How How we make hydrocarbons reactive? phosphate H2PO4 5. Phosphate group -Look at those hydrogen nuclei…are they held tightly? -compounds containing this group (organic phosphates) are typically acidic because those protons fall off into solution decreasing the pH. The oxygens of the phosphate will become negative when this happens. - Molecules that typically have this group are nucleic acids (DNA, RNA, nucleotides) and phosphlipids - Look at the picture above. When you see something connected to an “R”, the “R” is the organic molecule. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: cancan we make hydrocarbons reactivemore at biological temperatures? AIM:How How we make hydrocarbons reactive? sulfhydryl -SH 6. Sulfhydryl group - The sulfur is partial negative and the hydrogen partial positive for reasons you should know - This group is typically found in proteins - Molecules containing –SH are called thiols **Two sulfhydryl groups can interact forming a covalent bond known as a disulfide bridge in proteins. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: cancan we make hydrocarbons reactivemore at biological temperatures? AIM:How How we make hydrocarbons reactive? SUMMARY Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: cancan we make hydrocarbons reactivemore at biological temperatures? AIM:How How we make hydrocarbons reactive? Find the functional groups… You should be able to find one carbonyl group and five hydroxyl groups. What type of compound is this? Based solely on what you have learned thus far, you should respond by saying it is both an aldehyde because the carbonyl is at the end of a carbon skeleton and attached to a hydrogen, and an alcohol because of the hydroxyl groups… (Don’t worry about the red numbers…yet; this is glucose) Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: cancan we make hydrocarbons reactivemore at biological temperatures? AIM:How How we make hydrocarbons reactive? Find the functional groups… You should be able to find a carboxyl group (be careful, there is no carbonyl or hydroxyl group), an amino group and a sulfhydryl group. This is an amino acid. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: cancan we make hydrocarbons reactivemore at biological temperatures? AIM:How How we make hydrocarbons reactive? Draw an organic molecule containing an amino group and a carboxyl group. Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: cancan we make hydrocarbons reactivemore at biological temperatures? AIM:How How we make hydrocarbons reactive? ATP Adenosine Triphosphate What can we say about this molecule? 1. It is composed of a ribose sugar, three negative phosphates, and an adenine base 2. It’s an RNA nucleotide 3. Primary energy carrying molecule of the cell – fuel for proteins to do work / accelerate matter How is the energy stored in this molecule? Chapter and the Molecular Chapter43–- Carbon The Molecules of Cells Diversity of Life AIM: cancan we make hydrocarbons reactivemore at biological temperatures? AIM:How How we make hydrocarbons reactive? ATP Adenosine Triphosphate How is the energy stored in this molecule? - Look at the phosphates…what is their charge? - They are negative and hence repel each other. - Break the bond between phosphates via hydrolysis and…bam…the gun fires. ATP is a loaded gun. The phosphate will accelerate onto a protein.