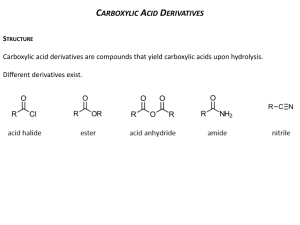
Carboxylic Acid Derivatives
advertisement

Substitution Reactions of Carboxylic Acid Derivatives Mechanism LINK Substitution at the sp2 hybridized carbonyl group employs a two-step additionelimination sequence as shown above. Relative to substitution at an sp3 hybridized carbon, the two-step additionelimination scheme of the carboxylic acid derivatives is more facile…. i.e. it has a lower energy barrier, due to the placement of the negative charge in the intermediate on an atom of higher electronegativity (oxygen). Nature frequently employs carboxylic acid derivatives, since these bonds are relatively easy to form and to cleave. Enzymes can catalyze both processes. Mechanistic Features of Peptide Bond Formation in the Ribosome LINK LINK Mechanistic Features of Peptide Bond Hydrolysis (in the active site of a peptidase enzyme) Triglyceride Acetyl CoA Carboxylic Acid Derivatives Acid Chloride + Amine → Amide Notice the inclusion of the tertiary amine (Et3N) to neutralize the HCl side product. Mechanism of Acid Chloride + Amine → Amide Notice that the amine nitrogen is functioning as a nucleophile, while the amide nitrogen is not (due to the resonance stabilization of the amide bond). Notice that the amine nitrogen is a stronger nucleophile than is the hydroxy group (due to higher basicity and nucleophilicity of nitrogen). Notice that the azide functionality (N3) is not nucleophilic. Notice greater reactivity of acyl chloride (RCOCl) than that of alkyl halide (RCH2X). Notice the use of pyridine in the example below as both a mild nucleophile, but also as a catalyst for the acylation process. Notice that the secondary amine is the nucleophile, while the tertiary nitrogen remains unreacted. 4-Dimethylaminopyridine (DMAP), used in the example below, is a particularly effective catalyst of the acylation process. Acid Chloride + Alcohol → Ester Acid Chloride + Hydride Source → Primary Alcohol Recall that nucleophilic hydride sources are boron or aluminum-based, while NaH is used almost exclusively as a base (not as a nucleophile) Notice the preferential reduction of the (more reactive) acid chloride over the (unreactive, under these conditions) ester functionalities. Acid Chloride + (2 eq) Carbanion → Tertiary Alcohol Anhydride + Amine → Amide Formation of an Acid Chloride from a Carboxylic Acid Ester To Acid Esters can be hydrolyzed by aqueous base (saponification). The mechanism is shown below. Note that the process involves formation of a tetraehedral intermediate and consumes a stoichiometric amount of base (since the product carboxylic acid is itself acidic and will thus be converted to its conjugate base under the basic reaction conditions. The hydrolysis of an ester under basic conditions is called saponification Notice that the ester is hydrolytically cleaved, but not the (unreactive) ether linkage. Notice that the ester linkage is cleaved, but not the (less reactive) cyclic amide (lactam) linkage. Notice that the ester linkage is hydrolyzed, but not the amide or the carbamate (RO-CO-NHR’). Notice that the one ester linkage is cleaved by hydroxide, but the four ether linkage are stable toward base. The hydrolysis of an ester can also occur under acidic conditions. Mechanism of Hydrolysis of Esters Under Acidic Conditions Enzyme-catalyzed Ester to Acid (cleavage of bonds other than the bonds to the carbonyl carbon) tert-Butyl esters (and also benzhydryl esters RCO2CHPh2) are cleaved upon treatment with strong acid, such as trifluoroacetic acid (TFA) shown below. Mechanism for acid-catalyzed cleavage of tert-Butyl esters Notice cleavage of the tert-butyl ester, but not the benzyl ester Notice cleavage of the tert-butyl ester, but not the methyl ester The selective cleavage of benzyl esters can be achieved by hydrogenolysis, as shown below. Notice cleavage of the benzyl ester, but not the methyl ester Notice cleavage of the benzyl ester, but not the tert-butyl esters Allyl esters can be cleaved by treatment with a catalytic amount of Pd0 and an appropriate nucleophile (to intercept the intermediate p-allyl palladium complex) Notice the selective cleavage of the allyl ester, but not the tert-butyl ester, or either of the two carbamates. Trichloroethyl esters are cleaved by treatment with zinc, as shown below Mechanism of cleavage Notice the selective cleavage of the trichloroethyl ester by zinc Ester to Amide Since esters are, in general, less reactive than more activated carboxylic acid derivatives (like acid chlorides or anhydrides), the reaction of esters with amines, for example, requires higher temperatures and longer reaction times. Notice that the amine nitrogen is the most nucleophilic heteroatom. Some esters, such as the pentafluorophenyl ester shown below, are more activated toward nucleophiles (due to their more electronegative nature) than are simple esters. Another common activated ester is the p-nitrophenyl ester shown below. This ester has a resonance stablized p-nitrophenolate anion as a leaving group. Ester to Ester The transformation of one ester into another can be catalyzed by acid or base, as shown in the examples below. 10-CSA = 10-camphorsulfonic acid = Acid to Ester The esterification of a carboxylic acid using excess of an alcohol and an acid-catalyst (such as para-toluenesulfonic acid shown, is called the Fischer esterification (also known as Fischer-Speier esterification). The mechanism of acid-catalyzed esterification is shown below. This is simply the reverse of the acidcatalyzed hydrolysis of esters shown earlier. One method for driving the reaction toward completion (in this equilibrium process) is to remove the product water by azeotropic distillation using a Dean-Stark apparatus shown below. The solvent is usually benzene or toluene, which forms an azeotrope with water, which, upon cooling, re-separates into two layers, water and solvent, with the water being more dense and sinking to the bottom of the trap as shown above. Thus the upper solvent layer is allowed to flow back into the reaction, while the lower layer of the distillate is removed via a stopcock. Notice that, under the acidic conditions of the Fischer esterification, that free amino groups become protonated to their conjugate acids, and thus do not function as nucleophiles in the coupling procedure. Acid to Ester (Reactions that do not involve attack on the carbonyl carbon) Acid to Amide (including the formation of peptides) Problems with conversion of the carboxylic acid to an acid chloride: 1) Requires extra step 2) Other functionality in molecule may not be compatible with thionyl chloride (SOCl2) reagent 3) In compounds where the a-carbon is stereogenic, this will likely lead to racemization due to enhanced formation of the enol form of the carbonyl. Acid to Amide via Carbodiimide Coupling Reagents However, the use of carbodiimides, by themselves, can still lead to epimerization of the a-carbon, as shown below. Additional Additives Can Reduce Epimerization: 1-Hydroxybenzotriazole (HOBt, 1) and 1-Hydroxy-7-azabenzotriazole (HOAt, 2) are common additives to the carbodiimide coupling reactions. These reagents rapidly react with the active intermediates, generating new intermediates that less susceptible to racemization. The use of an additive, like hydroxybenzotriazole, can reduce racemization and still allow efficient coupling. 1-Hydroxy-7-azabenzotriazole (HOAt) has the additional advantage of accelerating the reaction via an intramolecular assistance through the basic pyridine as shown below. Byproduct substituted urea can be difficult to remove from the reaction The use of the amino-functionalized substituted carbodiimide shown below can eliminate this problem (however, EDC is much more costly than DCC) Other Coupling Reagents BOP Reagent (Benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate) or pyBOP (Benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate) HATU 2-(7-Aza-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate Amide to Acid Enzymatic Link Hydride Reduction of Esters Reaction of Carboxylic Acid Derivatives with Organometallics Grignard Reagent + Ester→ Tertiary Alcohol Acid to Ketone Use of Weinreb’s Amide Allows Synthesis of Ketones from Acids Biological systems utilize anhydride-like linkage to store energy