Practice Problem - HCC Southeast Commons
advertisement

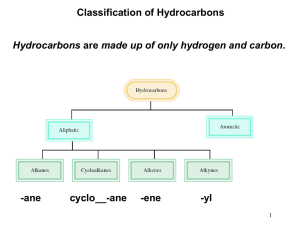
Chapter 3 Introduction • The structural theory is the basis of organic chemistry: Organic compounds can be grouped into families by their common structural features The members of a given family often have similar chemical and physical behavior • Hydrocarbons - are organic molecules consisting only of carbon (C) and hydrogen (H) atoms. Hydrocarbons Aliphatic Hydrocarbons Alkanes Alkenes Aromatic Hydrocarbons Alkynes I. Functional Groups A. Definition B. Types of Functional Groups A. Definition • Functional Group - is a group of atoms within a larger molecule that has a characteristic chemical reactivity • It reacts in a typical way, generally independent of the rest of the molecule The chemistry of every organic molecule, regardless of size and complexity, is determined by its functional groups. B. Types of Functional Groups • Table 3.1 lists a wide variety of functional groups. • The functional groups of a compound affect: • its reactions • its structure • its physical properties Functional Groups with Carbon-Carbon Multiple Bonds Functional Group with Carbon-Carbon Multiple Bonds: Alkene • Alkenes have a C-C double bond Functional Group with Carbon-Carbon Multiple Bonds: Alkyne • Alkynes have a C-C triple bond Functional Group with Carbon-Carbon Multiple Bonds: Arene • Arenes have special bonds that are represented as alternating single and double C-C bonds in a sixmembered ring Functional Groups with Carbon Singly Bonded to an Electronegative Atom Functional Group with Carbon Singly Bonded to an Electronegative Atom: Alkyl halide • Alkyl halides have a C bonded to a halogen (C-X) Functional Group with Carbon Singly Bonded to an Electronegative Atom: Alcohol • Alcohols have a C bonded to O of a hydroxyl group (C-OH) Functional Group with Carbon Singly Bonded to an Electronegative Atom: Ether • Ethers have two C’s bonded to the same O (C-O-C) Functional Group with Carbon Singly Bonded to an Electronegative Atom: Amine • Amines have a C bonded to N (C-N) Functional Group with Carbon Singly Bonded to an Electronegative Atom: Thiol • Thiols have a C bonded to SH group (C-SH) Functional Group with Carbon Singly Bonded to an Electronegative Atom: Sulfide • Sulfides have two C’s bonded to same S (C-S-C) Bonds are polar, with partial positive charge on C (+) and partial negative charge (-) on electronegative atom Functional Groups with Carbon-Oxygen Double Bond (Carbonyl Groups) • These compounds behave similarly but differ depending on the identity of the atoms attached on the carbonyl-group carbon. Functional Groups with Carbon-Oxygen Double Bond (Carbonyl Groups) • Aldehydes: have one hydrogen bonded to C=O • Ketones: have two C’s bonded to the C=O Functional Groups with Carbon-Oxygen Double Bond (Carbonyl Groups) • Carboxylic Acids: have -OH bonded to the C=O • Esters: have one ether-like C-O bonded to the C=O Functional Groups with Carbon-Oxygen Double Bond (Carbonyl Groups) • Amides: have one amine-like N bonded to the C=O • Acid chlorides: have a Cl bonded to the C=O Carbonyl C has partial positive charge (+) Carbonyl O has partial negative charge (-). Practice Problem: Identify the functional groups in each of the following molecules: Practice Problem: Propose structures for simple molecules that contain the following functional groups: a. Alcohol b. Aromatic ring c. Carboxylic acid d. Amine e. Both ketone and amine f. Two double bonds Practice Problem: Identify the functional groups in the following model of arecoline, a veterinary drug used to control worms in animals. Convert the drawing into a line-bond structure and a molecular formula (red = O, blue = N) II. Alkanes A. Alkane Isomers B. Alkyl Groups C. Naming Alkanes D. Properties of Alkanes Overview • Alkanes - are saturated aliphatic hydrocarbons. • They are compounds that contain only carbons and hydrogens (hydrocarbons), all connected exclusively by single bonds (saturated) • They are a large family of molecules • Alkanes - have the general formula CnH2n+2 where n is an integer A. Alkane Isomers • Isomers - are different compounds with the same molecular formula Isomers Constitutional Isomers Stereoisomers Enantiomers Diastereomers • For an alkane with less than three carbons, there is only one possible structure. • The molecular formula of an alkane with more than three carbons can give more than one structure • Straight-chain alkanes or normal alkanes - are compounds whose carbons are connected in a row. • The C’s are connected to no more than 2 other C’s • Branched-chain alkanes - are compounds whose carbon chains branch. • There are one or more C’s connected to 3 or 4 C’s • Constitutional Isomers - are isomers that differ in how their atoms are arranged in chains • They are isomers with different connectivity, i.e different order of attachment of their atoms • Constitutional isomers may have: Condensed Structures of Alkanes • An alkane can be represented in a brief form or in many types of extended form • A condensed structure does not show bonds but lists atoms, such as CH3CH2CH3 (propane) CH3C(CH3)2CH3 (2,2-dimethylpropane) Practice Problem: Draw structures of the five isomers C6H14 Practice Problem: Propose structures that meet the following descriptions a. Two isomeric esters with the formula C5H10O2 b. Two isomeric nitriles with the formula C4H7N Practice Problem: How many isomers are there with the following descriptions? a. Alcohols with the formula C3H8O b. Bromoalkanes with the formula C4H9Br B. Alkyl Groups • AlkyI group - is the partial structure that remains when a hydrogen atom is removed from an alkane - has the general abbreviation “R” (for Radical, an incomplete species or the “rest” of the molecule) Naming Alkyl groups • Replace -ane ending of alkane with -yl ending -CH3 is “methyl” from methane -CH2CH3 is “ethyl” from ethane • Combining an alkyl group with any of the functional groups gives the name of the compound Degree of a Carbon atom Degree (o) is applied to sp3 carbons only ! Degree (o) of a carbon is equal to the number of carbons attached directly to it. 1o primary 2o secondary 3o tertiary 4o quaternary Types of Alkyl groups Alkyl groups are classified by the connection site primary alkyl group (attached to 1oC) secondary alkyl group (attached to 2oC) tertiary alkyl group (attached to 3oC) quaternary alkyl group (attached to 4oC) Naming Alkyl groups: Common System • Free valence indicates carbon # 1 • Prefixes are necessary to indicate arrangement of carbons in structure: • n – unbranched chain and free valence is on 1o C • iso – one -CH3 on next to last carbon • sec – free valence on 2o C and used only for butyl • tert – free valence is on 3o C • neo – two -CH3’s next to the last carbon • iso – one -CH3 on next to last carbon • iso – one -CH3 on next to last carbon • sec – free valence on 2o C and used only for butyl • tert – free valence is on 3o C • iso – one -CH3 on next to last carbon • tert – free valence is on 3o C • neo – two -CH3’s next to the last carbon Degree of a Hydrogen atom Degree (o) of a hydrogen is the same as the o of carbon to which it is attached No such thing as a quaternary H ! Practice Problem: Draw the eight 5-carbon alkyl groups (pentyl isomers) Practice Problem: Identify the carbon atoms in the following molecules as primary, secondary, tertiary or quaternary Practice Problem: Identify the hydrogen atoms on the compounds shown as primary, secondary, or tertiary Practice Problem: Draw structures of alkanes that meet the following descriptions a. An alkane with two tertiary carbons b. An alkane that contains an isopropyl group c. An alkane that has one quaternary and one secondary carbon C. Naming Alkanes • The International Union of Pure and Applied Chemistry (IUPAC) devised a system of nomenclature that uses: Steps to naming alkanes1 1. Find the parent hydrocarbon a. Find the longest continuous chain of carbon atoms Steps to naming alkanes1 1. Find the parent hydrocarbon a. Find the longest continuous chain of carbon atoms b. If two chains have equal length, choose the main chain with more substituents Steps to naming alkanes2 2. Number the atoms in the main chain • The correct sequence is when the substituents have the lowest possible number Steps to naming alkanes3 3. Identify and number the substituents • Name and locate the substituents Steps to naming alkanes4 4. Write the name as a single word a. Use hyphens to separate the different prefixes, and use commas to separate numbers. b. Use multiplicity prefixes di-, tri-, tetra-, … b. Put the substituents in alphabetical order (Do not consider multiplier prefixes) Steps to naming alkanes5 5. Name a complex substituent as though it were itself a compound a. Name the complex substituent using the point of attachment as C#1 b. Put it in alphabetical order (numerical prefix is included) c. Set it off in parentheses Practice Problem: Give IUPAC names for the following compounds Practice Problem: Draw structures corresponding to the following IUPAC names a. 3,4-Dimethylnonane b. 3-Ethyl-4,4-dimethylheptane c. 2,2-Dimethyl-4-propyloctane d. 2,2,4-Trimethylpentane Practice Problem: Name the eight 5-carbon alkyl groups Practice Problem: Give the IUPAC name for the following hydrocarbon, and convert the drawing into skeletal structure. D. Properties of Alkanes • Alkanes are called paraffins (low affinity compounds) • They do not react as most chemicals, i.e. they are chemically inert to most lab reagents • However, they do react with O2, halogens and a few other substances Oxidation: Reaction with O2 • Alkanes will burn in a flame, producing carbon dioxide, water, and heat Alkane O2 D CO2 + H2O DH = - • The larger the value of DH, the less stable the alkane • If hydrocarbons do not contain the same amount of carbons, one must compare DH/CH2 rather than DH Halogenation • Alkanes react with Cl2 in the presence of light to replace H’s with Cl’s (not controlled) Alkane • X2 = Br2 or Cl2 X2 hn Alkyl halides + HX Physical Properties 1. Intermolecular Forces 2. Solubility 1. Intermolecular Forces • To a first approx. C-H and C-C bonds are nonpolar: • Dipole moments of alkanes are ~0 • Alkanes are nonpolar • The IMF’s for alkanes are van der Waals interactions (i.e induced-dipole-induced-dipole forces, London or dispersion forces) • For compounds of similar M.W., alkanes have lower b.p’s and m.p’s. • Boiling points and melting points increase as size of alkane increases. • This is due the presence of dispersion forces • Dispersion forces are weak intermolecular forces that arise due to transient nonuniform electron distribution (temporary molecular dipoles). • Increased branching lowers an alkane’s boiling point Branched-chain alkanes are more nearly spherical than straight-chain alkanes, thus have smaller surface areas, and consequently have smaller dispersion forces. Compounds Boiling Point Pentane 36.1 oC Isopentane 27.85 oC Neopentane 9.5oC Octane 125.7oC Isooctane 99.3oC 2. Solubility • “Like dissolves like” • Alkanes are nonpolar; they dissolve best in solvents which are either nonpolar or weakly polar # Carbons 4 ~ # Hydrophilic sites 1 III. Cycloalkanes A. Naming Cycloalkanes B. Cis-Trans Isomerism Overview • Cycloalkanes – are alicyclic (aliphatic cyclic) compounds • They are alkanes that have carbon atoms that form a ring • Their structure is shown as a regular polygon with the number of vertices equal to the number of C’s Simple Cycloalkanes • Cycloalkanes – are rings of -CH2- units. – have the general formula CnH2n where n is an integer Complex Cycloalkanes • Naturally occurring materials contain cycloalkane structures • Examples: chrysanthemic acid, prostaglandins, steroids (cyclopropane) (cyclopentane) (cyclohexane and cyclopentane) Properties of Cycloalkanes • Boiling points increase as ring size increases. • Melting points are affected by the shapes and the way that crystals pack so they do not change uniformly A. Naming Cycloalkanes • Cycloalkanes are also named by the rules devised by the International Union of Pure and Applied Chemistry (IUPAC). Steps to naming cycloalkanes1 1. Find the parent hydrocarbon a. Count the number of carbon atoms in the ring and the number in the largest substituent chain • • If # Cring = or > # Csubstituent, then it is named as an alkyl-substituted cycloalkane If # Cring < # Csubstituent, then it is named as an cycloalkyl-substituted alkane Steps to naming cycloalkanes2 2. Number the substituents and write the name • The correct sequence is when the substituents have the lowest possible number • For an alkyl- or halo-substituted cycloalkane, start at a point of attachment as C1 and number the substituents on the ring so that the second substituent has as low a number as possible. • When two or more substituents could potentially be assigned the same numbers, number them by alphabetical order. When two or more substituents could potentially be assigned the same numbers, number them by alphabetical order. Practice Problem: Give IUPAC names for the following cycloalkanes Practice Problem: Draw structures corresponding to the following IUPAC names a. 1,1-Dimethylcyclooctane b. 3-Cyclobutylhexane c. 1,2-Dichlorocyclopentane d. 1,3-Dibromo-5-methylcyclohexane Practice Problem: Name the following cycloalkane B. Cis-Trans Isomerism • In open-chain alkanes, free rotation is possible around C-C bonds because s bonds are cylindrically symmetrical: • In cycloalkanes, free rotation is limited by the ring structure • The common ring sizes (C3, C4, C5, C6, C7) are severely restricted in their molecular motions • Larger cycloalkanes have increasingly more rotational freedom • Rings have two “faces” and substituents are labeled as to their relative facial positions • There are two different 1,2-dimethyl-cyclopropane isomers: one with the two methyls on the same side (cis) of the ring one with the methyls on opposite sides (trans) Stereoisomers • Stereoisomers – are compounds with atoms connected in the same order but which differ in three-dimensional orientation • The terms “cis” and “trans” should be used to specify stereoisomeric ring structures • Constitutional isomers – are compounds with atoms connected in different order Practice Problem: Name the following substances, including the cis- or trans- prefix Practice Problem: Name the following substances, including the cis- or trans- prefix Practice Problem: Draw the structures of the following molecules a. trans-1-Bromo-3-methylcyclohexane b. cis-1,2-Dimethylcyclobutane c. trans-1-tert-Butyl-2-ethylcyclohexane Practice Problem: Name the following substances, including the cis- or trans- prefix (red-brown = Br) IV. Bicyclic Alkane Systems A. Naming Bicyclic Systems Overview • Bicycloalkanes – are alkanes containing two rings that share two carbon atoms • Bridgehead atoms – are the shared carbon atoms • Bridges – are the carbon chains connecting the bridgeheads • Bicycloalkanes – have the general formula CnH2n-2 where n is an integer • Spiroalkanes – are cycloalkanes in which two rings share only one carbon atom A. Naming Bicyclic Alkane Systems • The International Union of Pure and Applied Chemistry (IUPAC) has devised rules to name bicyclic alkane systems. Steps to naming bicycloalkanes1 1. Find the parent name • The parent name of a bicycloalkane is that of the unbranched alkane of the same number of carbon atoms as are in the bicyclic ring system 2. Numbering begins at one bridgehead carbon atom #1 #1 CH3 Steps to naming cycloalkanes2 3. Number the bridges in order of decreasing size • Longest bridge is written first • • Proceed along the longest bridge to the second bridgehead carbon, then along the next longest bridge back to the original bridgehead carbon, and so on until all carbon atoms are numbered. If there are two bridges of the same length, the correct sequence is when the substituents have the lowest possible number • If there are more than one substituents, give an alphabetical preference. Steps to naming cycloalkanes3 4. Show the bridge lengths in brackets • Count the number of carbons linking the bridgeheads and place them in decreasing order in brackets between the prefix bicyclo and the parent name and with periods separating each number. CH3 Bicyclo [3.2.0] heptane 7-methylbicyclo [4.2.0]octane Chapter 3