Kinetic Theory and Fluids
advertisement

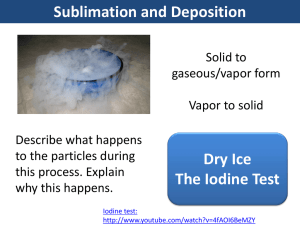
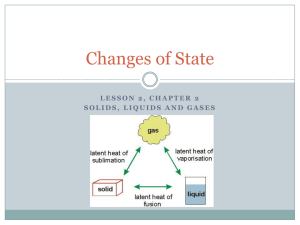
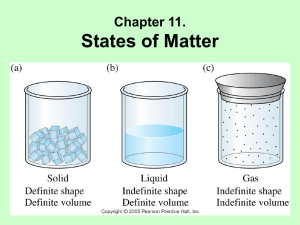
Kinetic Molecular Theory and the the Nature of Fluids A model for liquids/Evaporation Vapor Pressure Boiling Point Kinetic Molecular Theory States that that all substances are made of tiny particles (atoms and molecules) that are in constant motion The kinetic energy and motion of these particles can help us to understand the nature of fluids and phase changes A model for Liquids and gases- Kinetic Theory Liquids and Gases have kinetic energy---allows flow No attraction between gas particles Intermolecular attraction between liquid particles keeps them together Interplay between disruptive motions of particles in a liquid and attractions among particles determines physical properties of the liquid. Phase Changes using Kinetic Theory Solid Low KE IM Forces greater than KE so substance stays together Liquid Medium KE Gas Highest KE KE Forces overcome the IM forces to escape Density and Pressure Liquids much more dense than gas due to intermolecular attraction Increased pressure has little effect on liquids and solids volume Solids and Liquids are then called condensed states of matter Evaporation Conversion of liquid to gas = vaporization Most molecules don’t have enough kinetic energy to break free When vaporization is at the surface without boiling = evaporation During evaporation only those molecules with a certain minimum kinetic energy can escape from the surface of the liquid Further Evaporation Some escaping particle rebound back in off of air particles Heating increases kinetic energy which increases evaporation Removal of these higher energized particles leaves a lower average energy THEREFORE: evaporation is a cooling process Evaporation example Sweating uses evaporation as a cooling process Liquid on your skin takes heat energy Liquid evaporates taking that energy with it Leaves you with a lower temperature Vapor Pressure Vapor Pressure – measure of the force exerted by the gas leaving the surface of a liquid Over time they increase and particles condense ---eventually return to liquid state Vapor Pressure In a system of constant vapor pressure, a dynamic equilibrium exists between vapor and liquid Equilibrium because rate of evaporation = rate of condesation Vapor Pressure As temperature increases, vapor pressure increases Particles in liquid have increased kinetic energy More KE, able to escape surface Collide with “walls” Vapor pressure measured with a manometer Vapor increases, difference between levels increases Boiling Point Rate of evaporation increases as a liquid is heated KE increases as temperature increases When a liquid is heated to a temperature at which particles in the liquid have enough KE to vaporize, the liquid begins to boil. Boiling Point Boiling point- temperature at which the vapor pressure of the liquid is equal to the external pressure on the liquid Not all liquids have same boiling point Changes in altitude also affect boiling point Boiling Point Boiling similar to evaporation Particles with most KE rise to surface and break free Temperature of boiling liquid never rises above boiling point Escaping particles take growing energy with them Boiling Point Interesting fact: Burn from steam more severe than a burn from water Steam particles can carry more energy than water particles Collides with skin harder Boiling Point NORMAL BOILING POINT table ASSESSMENT What factors determine the physical properties of liquids? ASSESSMENT Explain how evaporation lowers the temperature of a liquid. ASSESSMENT What is vaporization? ASSESSMENT Define vapor pressure. ASSESSMENT What is a normal boiling point?