Alkyl halides and alcohols
Alkyl halides
Nucleophilic substitution and elimination reactions
© E.V. Blackburn, 2011
Alkyl halides - industrial sources
HCl
H C C H H
2
C=CHCl
HgCl
2 vinyl chloride
H H
H vinyl
© E.V. Blackburn, 2011
Alkyl halides - industrial sources
HCl
H C C H H
2
C=CHCl
HgCl
2 vinyl chloride
Cl
2
H
2
C=CH
2
H
2
C=CHCl
500 o
CH
3
Cl + Hg
2
F
2
CH
3
F + Hg
2
Cl
2
CCl
4
+ SbF
3
CCl
2
F
2
Freon-12
© E.V. Blackburn, 2011
Preparation from alcohols
HX
R-OH R-X or PX
3 or SOCl
2
SOCl
2
- thionyl chloride
RCH
2
OH + SOCl
2
RCH
2
Cl + HCl +SO
2
© E.V. Blackburn, 2011
Halogenation of hydrocarbons
X
2
/h
R-H RX
CH
3
Br
2 h
CH
2
Br lachrymatory
© E.V. Blackburn, 2011
Addition of HX to alkenes
C C
HX
C C
H X
© E.V. Blackburn, 2011
Addition of halogens to alkenes and alkynes
X
2
C C C C
X X
C C
2X
2
X X
C C
X X
© E.V. Blackburn, 2011
Finkelstein reaction acetone
R-X + NaI R-I + NaX soluble insoluble
© E.V. Blackburn, 2011
Nucleophilic substitution reactions
The halide ion is the conjugate base of a strong acid. It is therefore a very weak base and little disposed to share its electrons.
When bonded to a carbon, the halogen is easily displaced as a halide ion by stronger nucleophiles - it is a good leaving group .
The typical reaction of alkyl halides is a nucleophilic substitution:
R-X + Nu R-Nu + X
the leaving
group
© E.V. Blackburn, 2011
Nucleophiles
• reagents that seek electron deficient centres
• negative ions or neutral molecules having at least one unshared pair of electrons
-
H
3
C C C + CH
3
-Br H
3
C C C CH
3
+ Br
-
H
3
C O
H
+ CH
3
-I H
3
C
+
O
CH
3
H
+ I
nucleophile leaving group
© E.V. Blackburn, 2011
Leaving groups
• a substituent that can leave as a weakly basic molecule or ion
Nu
CN
-
Cl
-
Ph
3
P:
+ L
Nu L
Br
NC Br
OH
2
+
Cl OH
2
Br PH
3
P Br
Nu
NC
Cl
+
Ph
3
P
+ L:
+ Br
-
+ H
2
O
+ Br
-
© E.V. Blackburn, 2011
Nucleophilic substitution
CH
3
OH
-
CH
3
OH + Br
-
A knowledge of how reaction rates depend on reactant concentrations provides invaluable information about reaction mechanisms. What is known about this reaction?
© E.V. Blackburn, 2011
Nucleophilic substitution
CH
3
OH
-
CH
3
OH + Br
-
[CH
3
Br]
I
0.001 M
0.002 M
0.002 M
[OH ]
I
1.0 M
1.0 M
2.0 M initial rate
3 x 10 -7 mol
L -1
s -1
6 x 10 -7 mol
L -1
s -1
1.2 x 10 -6 mol
L -1
s -1 rate a
[CH
3
Br][OH ] rate = k[CH
3
Br][OH ]
© E.V. Blackburn, 2011
Order - a summary
The order of a reaction is equal to the sum of the exponents in the rate equation.
Thus for the rate equation rate = k[A] m [B] n , the overall order is m + n .
The order with respect to A is m and the order with respect to B is n .
© E.V. Blackburn, 2011
Nucleophilic substitution
CH
3
CH
3
-C-CH
3
+ OH
-
Br
CH
3
CH
3
-C-CH
3
OH
+ Br
-
[(CH
3
)
3
CBr]
I
0.001 M
0.002 M
0.002 M
[OH ]
I
1.0 M
1.0 M
2.0 M initial rate
4 x 10 -7 mol
L -1
s -1
8 x 10 -7 mol
L -1
s -1
8 x 10 -7 mol
L -1
s -1 rate a
[(CH
3
)
3
CBr][OH ] 0 rate = k[(CH
3
)
3
CBr]
© E.V. Blackburn, 2011
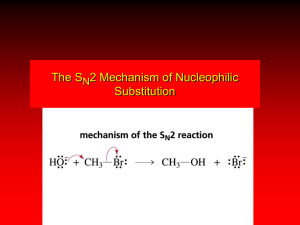
OH
-
The S
N
2 mechanism
CH
3
OH
-
CH
3
OH + Br
-
Br rate = k[CH
3
Br][OH ]
-
HO
-
Br
HO
+ Br
-
References of interest:
E.D. Hughes, C.K. Ingold, and C.S. Patel, J. Chem. Soc ., 526 (1933)
J.L. Gleave, E.D. Hughes and C.K. Ingold, J. Chem. Soc ., 236 (1935)
© E.V. Blackburn, 2011
OH
-
Stereochemistry of the S
N
2 reaction
Br
C
6
H
13
H
H
3
C
Br
(-)-2-bromooctane
[ a
] = -34.6
o
-
HO
C
6
H
13
H
H
3
C
OH
(-)-2-octanol
[ a
] = -9.9
o
-
Br
HO
+ Br
-
C
6
H
13
HO
H
CH
3
(+)-2-octanol
[ a
] = +9.9
o
© E.V. Blackburn, 2011
Stereochemistry of the S
N
2 reaction
C
6
H
13
NaOH
C
6
H
13
Br
H
S
N
2
HO
H
H
3
C
(-)-2-bromooctane
CH
3
(+)-2-octanol
[ a
] = -34.6
o
[ a
] = +9.9
o optical purity = 100%
A Walden inversion.
P. Walden, Uber die vermeintliche optische Activät der
Chlorumarsäure und über optisch active Halogenbernsteinsäre , Ber.
, 26, 210 (1893)
© E.V. Blackburn, 2011
The S
N
1 mechanism
CH
3
CH
3
-C-CH
3
+ OH
-
Br
CH
3
CH
3
-C-CH
OH rate = k[(CH
3
)
3
CBr]
3
+ Br
-
1.
H
3
CH
3
C C CH
3
Br slow
CH
3
2.
H
3
C C
+
CH
3
+ OH
-
H
3
CH
3
C C
+
CH
3 fast
H
3
+ Br
-
CH
3
C C
OH
CH
3
© E.V. Blackburn, 2011
Carbocations
G.A. Olah, J. Amer. Chem. Soc., 94, 808 (1972)
CH
3
CH
3
+
CH
3
CH
2
+
CH
3
CHCH
+
3
CH
3
CCH
+
3
1 o
2 o
3 o sp
2
© E.V. Blackburn, 2011
Carbocation stability
R
R C
R
+
3 o
> R C
H
+
R
2 o
H
> R C
H
+
1 o
> H C
H
+
H
Hyperconjugation stabilizes the positive charge.
H
H
H
H
H
© E.V. Blackburn, 2011
Stereochemical consequences of a carbocation
1.
H
3
CH
3
C C CH
3
Br slow
H
3
CH
3
C C
+
CH
3
+ Br
-
C
6
H
13
H
H
3
C
Br
(-)-2-bromooctane
[ a
] = -34.6
o
OH
-
H
2
O
S
N
1
?
© E.V. Blackburn, 2011
Stereochemical consequences of a carbocation
1.
H
3
CH
3
C C CH
3
Br slow
C
6
H
13
H
H
3
C
Br
(-)-2-bromooctane
[ a
] = -34.6
o
OH
-
H
2
O
S
N
1
H
3
CH
3
C C
+
CH
3
+ Br
-
(+)-C
6
H
13
CHOHCH
3 reduced optical purity
Why?
© E.V. Blackburn, 2011
Stereochemical consequences of a carbocation
C
6
H
13 H
H
2
O
X
-
HO
C
6
H
13
H
+
CH
3
CH
3 inversion predominates retention
© E.V. Blackburn, 2011
Carbocation rearrangements
(CH
3
)
3
CCH
2
Br
C
2
H
5
O
-
S
N
2
(CH
3
)
3
CCH
2
OC
2
H
5
Williamson ether synthesis
C
2
H
5
OH
S
N
1
(CH
3
)
2
CCH
2
CH
3
OC
2
H
5
+
(CH
3
)
2
C=CHCH
3 a rearrangement and elimination
© E.V. Blackburn, 2011
Carbocation rearrangements
CH
3
CH
2
CH
+
2
CH
+
2
CH
3
CH
2
CHCH
3
1 o
2 o
+
H
C C
+ H
+
R
C C
+ R
1,2 hydride and alkyl shifts
© E.V. Blackburn, 2011
Carbocation rearrangements
(CH
3
)
3
CCH
2
Br (CH
3
)
3
+
CCH
2
H
3
C
CH
3
H
+
CH
3
H
CH
3
H
3
C CH
+
2
CH
3
C
2
H
5
OH
CH
3
H
3
C CH
2
CH
3
+
H
OC
2
H
5
CH
3
H
3
C CH
+
2
CH
3
-H
+
CH
3
H
3
C CH
2
CH
3
+
H
OC
2
H
5
CH
3
H
3
C CH
2
CH
3
OC
2
H
5
© E.V. Blackburn, 2011
OH
-
Steric effects in the S
N
2 reaction
-
-
Br HO Br
HO
+ Br
-
Look at the transition state to see how substituents might affect this reaction.
-
HO
-
Br
© E.V. Blackburn, 2011
Steric effects in the S
N
2 reaction
-
HO
-
Br
The order of reactivity of RX in these S
N
2 reactions is
CH
3
X > 1 o > 2 o > 3 o
© E.V. Blackburn, 2011
Steric effects in the S
N
2 reaction
RBr + I
-
RI + Br
reactivity
CH
3
Br >
150
CH
3
CH
2
Br
1
> (CH
3
)
2
CHBr
0.01
> (CH
3
)
3
CBr
0.001
I
-
-
Br
I
-
-
Br
I
-
-
Br I
-
-
Br
© E.V. Blackburn, 2011
Structural effects in S
N
1 reactions
3 o > 2 o > 1 o > CH
3
X
R-X
+
-
R X R
+
+ X
-
HCO
2
H
RBr + H
2
O ROH + HBr
(CH
3
)
3
CBr > (CH
3
)
2
CHBr > CH
3
CH
2
Br > CH
3
Br
100,000,000 45 1.7 1
© E.V. Blackburn, 2011
Nucleophilicity
Rates of S
N
2 reactions depend on concentration and nucleophilicity of the nucleophile.
A base is more nucleophilic than its conjugate acid:
CH
3
Cl + H
2
O
CH
3
OH
2
+
CH
3
Cl + HO -
CH
3
OH slow fast
The nucleophilicity of nucleophiles having the same nucleophilic atom parallels basicity:
RO > HO >> RCO
2
> ROH >H
2
O
© E.V. Blackburn, 2011
Nucleophilicity
When the nucleophilic atoms are different, their relative strengths do not always parallel their basicity.
In protic solvents, the larger the nucleophilic atom, the better:
I > Br > Cl > F -
In protic solvents, the smaller the anion, the greater its solvation due to hydrogen bonding. This shell of solvent molecules reduces its ability to attack.
© E.V. Blackburn, 2011
Nucleophilicity
Aprotic solvents tend to solvate cations rather than anions. Thus the unsolvated anion has a greater nucleophilicity in an aprotic solvent.
© E.V. Blackburn, 2011
Polar aprotic solvents
O
H N
H
3
C
CH
3
N,N -dimethylformamide
DMF
(H
3
C)
2
N
O
P N(CH
3
)
2
N(CH
3
)
2 hexamethylphosphoramide
HMPA
O
H
3
S
C CH
3 dimethyl sulfoxide
DMSO
These solvents dissolve ionic compounds.
© E.V. Blackburn, 2011
Solvent polarity
Cl
-
H
H
H
I Cl
-
-
I more polar transition state less solvated than reagents
A protic solvent will decrease the rate of this reaction and the reaction is 1,200,000 faster in DMF than in methanol.
© E.V. Blackburn, 2011
R-X
Solvent polarity
+
-
R X R
+
+ X
less polar more polar greater stabilization by polar solvent
The transition state is more polarized.
Therefore the rate of this reaction increases with increase in solvent polarity.
A protic solvent is particularly effective as it stabilizes the transition state by forming hydrogen bonds with the leaving group.
© E.V. Blackburn, 2011
Solvent polarity
Explain the solvent effects for each of the following second order reactions: a) 131 I + CH
3
I
CH
3
131 I + I -
Relative rates: in water, 1; in methanol, 16; in ethanol, 44 b) ( n -C
3
H
7
)
3
N + CH
3
I
( n -C
3
H
7
)
3
N + CH
3
I -
Relative rates: in n -hexane, 1; in chloroform, 13 000
© E.V. Blackburn, 2011
Leaving group ability
Weak bases are good leaving groups.
They are better able to accommodate a negative charge and therefore stabilize the transition state.
Thus I is a better leaving group than Br .
I > Br > Cl > H
2
O > F > OH -
© E.V. Blackburn, 2011
S
N
1 v S
N
2
S
N
1 S
N
2 kinetics: 1st order second order reactivity: 3 o > 2 o > 1 o > CH
3
X CH
3
X > 1 o > 2 o > 3 o rearrangements no rearrangements partial inversion inversion of configuration eliminations possible
© E.V. Blackburn, 2011
Functional group transformations using S
N
2 reactions
R = Me, 1 o , or 2 o
CN
-
R-CN nitrile
'R
C
C
-
R C C R' alkyne
© E.V. Blackburn, 2011
ROH + HX - an S
N reaction
ROH + HX RX + H
2
O
HX: HI > HBr > HCl
ROH: 3 o > 2 o > 1 o < CH
3
OH
HBr or
CH
3
CHCH
3
OH
NaBr/H
2
SO
4
CH
3
CHCH
3
Br
© E.V. Blackburn, 2011
Experimental facts
1. The reaction is acid catalyzed
2. Rearrangements are possible
CH
3
H
H
3
C C C
H OH
CH
3
HCl
CH
3
H
H
3
C C C
Cl H
CH
3
3. Alcohol reactivity is 3 o > 2 o > 1 o < CH
3
OH
© E.V. Blackburn, 2011
The mechanism
1. ROH + HX
+
ROH
2
+ X
-
+
2. ROH
2
+
R + H
2
O
+ -
3. R + X RX
© E.V. Blackburn, 2011
Reaction of primary alcohols with HX
1. ROH + HX
1 o
+
ROH
2
+ X
-
+
2. ROH
2
+ X
-
-
+
X R OH
2
RX + H
2
O
S
N
2
HX: HI > HBr > HCl
© E.V. Blackburn, 2011
© E.V. Blackburn, 2011