I. Alkynes
advertisement

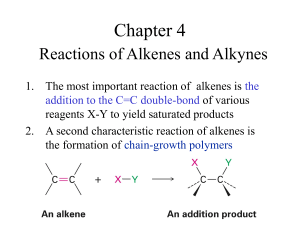
Chapter 8 Introduction • An alkyne is a hydrocarbon that contains a carbon-carbon triple bond • Acetylene is the simplest alkyne. • It is produced industrially from methane by high temperature decomposition (pyrolysis) • It is the starting material of many organic molecules I. Alkynes: An Overview A. Electronic Structure B. Naming Alkynes A. Electronic Structure Carbon-carbon triple bond results from: – sp hybrid orbital on each C forming a s bond and – unhybridized py and pz orbitals forming a p bond – The remaining sp orbitals form bonds to other atoms at 180º to C-C triple bond. – The triple bond is shorter and stronger than single or double bond – The triple bond is shorter and stronger than single or double bond • Breaking a p bond in acetylene (HCCH) requires 318 kJ/mole (in ethylene it is 268 kJ/mole) B. Naming Alkynes • Like alkanes and alkenes, alkynes are named according to the system devised by the International Union of Pure and Applied Chemistry (IUPAC). Steps to naming alkynes1 a. Find the longest continuous carbon chain containing the triple bond b. Name using the suffix –yne to indicate an alkyne. – The position of the triple bond is indicated by giving the number of the first alkyne carbon c. Numbering of chain with triple bond is set so that the smallest number possible includes the triple bond Steps to naming alkynes2 d. If more than one triple bond is present: – Indicate the position of each and use the suffixes diyne, -triyne, … – A compound with two triple bonds is a diyne – A triyne has three triple bonds Steps to naming alkynes3 e. If a triple and a double bond are present: – Name the compound an enyne – An enyne has a double bond and triple bond – Number from chain that ends nearest a double or triple bond - double bond is preferred if both are present in the same relative position • Alkynes as substituents are called “alkynyl” Practice Problem: Name the following compounds: Practice Problem: There are seven isomeric alkynes with the formula C6H10. Draw and name them II. Synthesis of Alkynes A. Elimination Reactions of Dihalides B. Alkylation of Acetylide Anions A. Elimination Reactions of Dihalides • Treatment of a 1,2 dihaloalkane (vicinal dihalide) with KOH or NaNH2 produces a two-fold elimination of HX and formation of an alkyne – Intermediate is a vinyl halide – Vicinal dihalides are available from addition of bromine or chlorine to an alkene – Vinyl halides give alkynes when treated with strong base. Dehydrohalogenation of Vicinal dihalides Dehydrohalogenation of vicinal dihalides results in twofold elimination (loss) of HX and formation of an alkyne – uses strong base (KOH or NaNH2) – Intermediate is a vinyl halide B. Alkylation of Acetylide Anions III. Reactions of Alkynes A. Addition of HX and X2 B. Hydration of Alkynes C. Reduction of Alkynes D. Oxidative Cleavage of Alkynes III. Reactions of Alkynes E. Alkyne Acidity: Formation of Acetylide Anions F. Alkylation of Acetylide Anions G. An Introduction to Organic Synthesis Introduction • Addition reactions of alkynes are similar to those of alkenes • Alkynes react with many electrophiles to give useful products by addition A. Addition of HX and X2 • Addition of excess H-X to an alkyne gives a dihalide product – Regiochemistry is Markovnikov (X attaches to the more highly substituted sp carbon and H adds to the less highly substituted side) Addition of X2 (where X = Br or Cl) • Addition of excess X2 to an alkyne gives a tetrahalide product. • Initial addition of X2 (Br2 or Cl2) to an alkyne gives trans intermediate Addition of HX to Alkynes Involves Vinylic Carbocations • Addition of H-X to alkyne should produce a vinylic carbocation intermediate – Secondary vinyl carbocations form less readily than primary alkyl carbocations – Primary vinyl carbocations probably do not form at all • Nonethelss, H-Br can add to an alkyne to give a vinyl bromide if the Br is not on a primary carbon • Vinylic carbocations are less stable than similarly substituted alkyl carbocations. – Vinyl carbocations have sp-hybridized carbons and thus lack stabilizing hyperconjugative interactions – Stability of carbocations: 3º alkyl > 2º alkyl > 1º alkyl ~ 2º vinyl > +CH3 ~ 1º vinyl Addition of HX and X2: Summary • Addition of excess H-X to an alkyne gives a dihalide – Markovnikov Regiochemistry • Addition of excess X2 to an alkyne gives a tetrahalide product. Practice Problem: What products would you expect from the following reactions? B. Hydration of Alkynes • Hydration of alkynes is the addition H-OH to an alkyne – Mercury (II) catalyzes Markovnikov oriented addition – Hydroboration-oxidation gives the non-Markovnikov product Mercury(II)-Catalyzed Hydration of Alkynes • Mercuric ion (as the sulfate) is a Lewis acid catalyst that promotes addition of water in Markovnikov orientation – The immediate product is a vinylic alcohol, or enol, which spontaneously rearranges to a ketone – Alkynes do not react with aqueous protic acids Keto-enol Tautomerism • Isomeric compounds that can rapidily interconvert by the movement of a proton are called tautomers and the phenomenon is called tautomerism • Enols rearrange to the isomeric ketone by the rapid transfer of a proton from the hydroxyl to the alkene carbon • The keto form is usually so stable compared to the enol that only the keto form can be observed Mechanism of Mercury (II) catalyzed Hydration • Addition of Hg(II) to alkyne gives a vinylic carbocation intermediate • Water adds and loses a proton • A proton from aqueous acid replaces Hg(II) Hydration of Unsymmetrical Alkynes • Unsymmetrically substituted internal alkyne: – If the alkyl groups at either end of the C-C triple bond are not the same, both products can form and this is not normally useful Hydration of Unsymmetrical Alkynes Terminal alkyne – If the triple bond is at the first carbon of the chain (then H is what is attached to one side) this is called a terminal alkyne – Hydration of a terminal alkyne always gives the methyl ketone, which is useful Mercury(II)-Catalyzed Hydration: Summary H-OH adds to an alkyne to give an enol which converts to ketone – Markovnikov regiochemistry – Mercury-containing vinylic carbocation intermediate Practice Problem: What product would you obtain by hydration of the following alkynes? Practice Problem: What alkynes would you start with to prepare the following ketones? Hydroboration/Oxidation of Alkynes • BH3 (borane) adds to alkynes to give a vinylic borane • Oxidation with H2O2 produces an enol that converts to the ketone or aldehyde • Process converts alkyne to ketone or aldehyde with Non-Markovnikov orientation • This is opposite to mercuric ion catalyzed hydration Comparison of Hydration of Terminal Alkynes • Hydroboration/oxidation converts terminal alkynes to aldehydes because addition of water is non-Markovnikov • Mercury(II) catalyzed hydration converts terminal alkynes to methyl ketones Hydroboration-oxidation: Summary Borane adds to an alkyne to give a vinylic borane which is then oxidized by alkaline H2O2 to give an enol which converts to ketone or aldehyde – Non-Markovnikov regiochemistry Practice Problem: What alkyne would you start with to prepare each of the following compounds by a hydroboration/oxidation reaction? C. Reduction of Alkynes • Reduction of alkynes is the addition H-H to an alkyne – Complete Catalytic hydrogenation using a metal catalyst (Pd/C) or – Partial Catalytic hydrogenation using Lindlar catalyst – Reduction with Lithium/ammonia Catalytic Hydrogenation • Addition of H2 over a metal catalyst (such as palladium on carbon, Pd/C) converts alkynes to alkanes (complete reduction) – The addition of the first equivalent of H2 produces an alkene intermediate, which is more reactive than the alkyne so the alkene is not observed Conversion of Alkynes to cis-Alkenes • Addition of H2 using the Lindlar catalyst (chemically deactivated palladium on calcium carbonate) produces a cis alkene – The two hydrogens add syn (from the same side of the triple bond) Conversion of Alkynes to trans-Alkenes • Alkynes are reduced to trans alkenes with sodium or lithium in liquid ammonia – Anhydrous ammonia (NH3) is liquid below -33 ºC – Alkali metals dissolve in liquid ammonia and function as reducing agents • The reaction involves a radical anion intermediate Reduction of Alkynes : Summary • Addition of H2 over a metal catalyst (Pd/C) to an alkyne produces an alkane • Addition of H2 using the Lindlar catalyst produces a cis alkene – syn stereochemistry • Alkynes are reduced to trans alkenes with sodium or lithium in liquid ammonia – a radical anion intermediate – anti stereochemistry Practice Problem: Using any alkyne needed, how would you prepare the following alkenes? a) trans-2-Octene b) cis-3-Heptene c) 3-Methyl-1-pentene D. Oxidative Cleavage of Alkynes • Strong oxidizing reagents (O3 or KMnO4) cleave – internal alkynes, producing two carboxylic acids – terminal alkynes, producing a carboxylic acid and carbon dioxide • Neither process is useful in modern synthesis. – Historically, these were used to elucidate structures because the products indicate the structure of the alkyne precursor Oxidative Cleavage of Alkynes: Summary Practice Problem: Propose structures for alkynes that give the following products on oxidative cleavage by KMnO4: E. Alkyne Acidity: Formation of Acetylide Anions • Reaction of strong anhydrous bases (Na+-NH2) with a terminal alkyne produces an acetylide ion – Terminal alkynes are relatively acidic • Terminal alkynes are weak Brønsted acids (pKa ~ 25) – Alkenes and alkanes are much less acidic. • Acetylide anions are more stable than either alkyl anions or vinylic anions because: – They have sp-hybridized carbon and their negative charge is in a hybrid orbital with 50% s character, allowing the charge to be closer to the nucleus. Formation of Acetylide Anions: Summary • Reaction of strong anhydrous bases (Na+-NH2) with a terminal alkyne produces an acetylide ion Practice Problem: The pKa of acetone, CH3COCH3, is 19.3. Which of the following bases is strong enough to deprotonate acetone? a) KOH (pKa of H2O =15.7) b) Na+ -CΞCH (pKa of C2H2 =25) c) NaHCO3 (pKa of H2CO3 = 6.4) d) NaOCH3 (pKa of CH3OH = 15.6) F. Alkylation of Acetylide Anions • Reaction of an acetylide anion with a primary alkyl halide produces a larger alkyne – Acetylide anions can react as nucleophiles as well as bases – Acetylide anions can displace a halide ion from a 1o alkyl halide Limitations of Alkylation of Acetylide Ions Reactions only are efficient with 1º alkyl bromides and alkyl iodides • • Acetylide anions can behave as bases as well as nucelophiles Reactions with 2º and 3º alkyl halides gives dehydrohalogenation, converting alkyl halide to alkene Alkylation of Acetylide Anions: Summary • Reaction of an acetylide anion with a primary alkyl halide produces a larger alkyne Practice Problem: Show the terminal alkyne and alkyl halide from which the following products can be obtained. If two routes look feasible, list both: Practice Problem: How would you prepare cis-2-butene starting from propyne, an alkyl halide, and any other reagents needed? This problem can’t be worked in a single step. You’ll have to carry out more than one reaction. G. An Introduction to Organic Synthesis Organic synthesis may be used to – produce new molecules that are needed as drugs or materials – design, test and improve efficiency and safety for making known molecules – test ideas and methods, answering challenges Synthesis as a Tool for Learning Organic Chemistry • In order to propose a synthesis, one must be familiar with reactions: – – – – What they begin with What they lead to How they are accomplished What the limitations are • A synthesis combines a series of proposed steps to go from a defined set of reactants to a specified product Strategies for Synthesis 1. Compare the target and the starting material 2. Consider reactions that efficiently produce the outcome. 3. Look at the product and think of what can lead to it Example – Problem: prepare octane from 1-pentyne – Strategy: use acetylide coupling Practice Problem: Beginning with 4-octyne as your only source of carbon and using any inorganic reagents necessary, how would you synthesize the following compounds? a) Butanoic acid b) cis-4-Octene c) 4-Bromooctane d) 4-Octanol (4-hydroxyoctane) e) 4,5-Dichlorooctane Practice Problem: Beginning with acetylene and any alkyl halides needed, how would you synthesize the following compounds? a) Decane b) 2,2-Dimethylhexane c) Hexanal d) 2-Heptanone Chapter 8 Addition of HX and X2: Summary Hydration of Alkynes Mercury (II) catalyzes Markovnikov oriented addition Hydroboration-oxidation gives the non-Markovnikov product Reduction of Alkynes : Summary Oxidative Cleavage of Alkynes: Summary Formation of Acetylide Anions: Summary Alkylation of Acetylide Anions: Summary • Reaction of an acetylide anion with a primary alkyl halide produces a larger alkyne Alkylation of Acetylide Anions: Summary • Reaction of an acetylide anion with a primary alkyl halide produces a larger alkyne