Acid-base balance and its disorders
advertisement
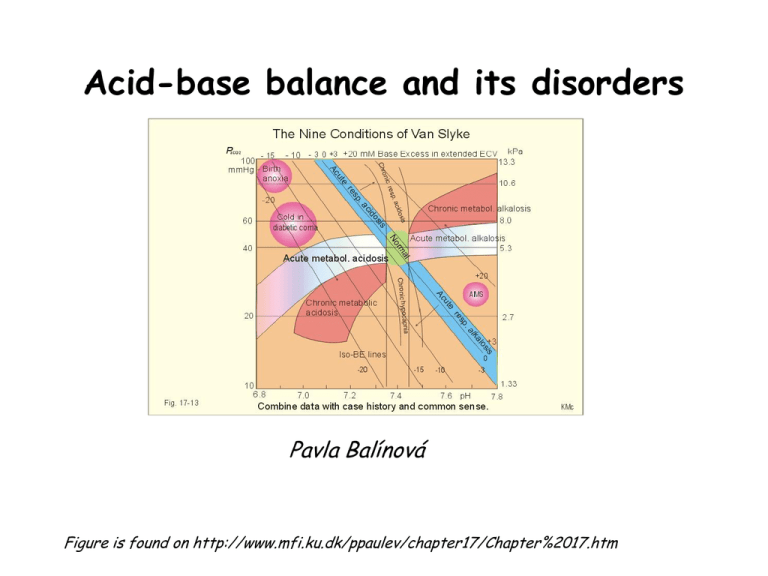
Acid-base balance and its disorders Pavla Balínová Figure is found on http://www.mfi.ku.dk/ppaulev/chapter17/Chapter%2017.htm Definitions • Acid (HA) is defined as a compound that can release a proton (H+) • Acidosis (acidaemia) is defined as a disorder with accumulation of acids in the extended ECV. The pH in the arterial blood is < 7.35 • Base (B-) can bind H+ • Alkalosis (alkalaemia) is defined as a condition with accumulation of bases in the extended ECV. The pH of the arterial blood is › 7.45 • Buffer is a mixture of compounds which have the ability to absorb small amounts of H+ or OH- with very little change of pH. pH = pK + log cs / cA Proton concentration and pH Normally, the [H+] of arterial blood of humans is maintained by the lungs, kidneys and liver within the range of 40 5 nM, corresponding to a pH of 7.35 - 7.45. pH = - log (40 x 10-9 mol/L ) = 7.4 A pH of 6.8 - 6.9 is not sustainable for long, and the patient is dying in a state of coma. Figure is found on http://www.mfi.ku.dk/ppaulev/chapter17/Chapter%2017.htm Production of acids ● CO2 is a potential acid as H2CO3, and because the lungs eliminate it, it is called a volatile acid. Production of CO2 is up to 24 mol daily. ● Non-volatile acids: a) organic acids are continually produced as a by-product of metabolism: - anaerobic glycolysis in muscles and ery → lactic acid → lactate + H+ - ketogenesis → acetoacetic acid → acetoacetate + H+ → β-hydroxybutyric acid → β-hydroxybutyrate + H+ - lipolysis → TAG → 3 FA + glycerol + 3 H+ - urea synthesis in liver: CO2 + 2 NH4 → urea + H2O + 2 H+ Under normal conditions, these acids are completely metabolized to CO2 and H2O. They have no effect on proton balance. b) inorganic acids: excretion by kidneys H2SO4 → HSO4- + H+ H3PO4 → HPO42- + H+ Note: H+ are also released from acids in the diet e. g. citric acid, ascorbic acid Consumption of acids (protons) • gluconeogenesis: 2 lactate + 2 H+ → Glc • oxidation of neutral AA and Glu and Asp The body maintains ECF physiologic pH by buffers • • • • Bicarbonate buffer HCO3- / CO2 Hemoglobin (Hb) – in ery Plasma proteins (mainly albumin) Phosphate buffer HPO42- / H2PO4- (53%) (35%) (7%) (3%) NH3/NH4+ and HPO42-/H2PO4- are the most important urinary buffer systems. About 30 mmol of NH4+ is excreted in the daily urine, but the excretion is controlled during acid-base disorders. HCO3-/CO2 system is an effective open buffer system HCO3- and CO2 are present in ratio of about 20 : 1. CO2 is dissolved in the plasma and it is constantly exchanging with CO2 in the gas phase of the alveoli of the lungs. Henderson-Hasselbach equation for HCO3-/CO2 system: pH = pK + log [HCO3-] / [H2CO3] pH = pK + log [HCO3-] / pCO2 x α pH = 6.1 + log 24 / 40 x 0.03 (pCO2 = 40 mmHg →factor α = 0.03) pH = 6.1 + log 20 pH = 6.1 + 1.3 = 7.4 Conversion: 1 kPa = 7.5 mmHg 1 mmHg = 133.22 Pa CO2 (pCO2) elimination is controlled by lungs (respiratory system). It takes 1 – 3 min to respond to changes in pH and effect changes in the pH. ↑ ventilation → ↓ pCO2 → alkalinization ↓ ventilation → ↑ pCO2 → acidification HCO3- elimination is controlled by kidneys. It takes several hours to days for urinary system to compensate for changes in pH. Liver: CO2 + 2 NH4 → urea + 2 H+ + H2O NH4+ + Glu → Gln + H2O Laboratory analysis of ABB state • Determination of pH, HCO3-, pCO2, pO2 and BE • Determination of concentration of cations (Na+, K+, Ca2+, Mg2+), concentration of anions (Cl-, lactate) and metabolites (urea, creatinine, ketone bodies) Normal values of: • HCO3- = 22 – 26 mmol/L • BE = from – 2.5 to + 2.5 mmol/L BE (base excess) is defined as the amount of acid that would be added to blood to titrate it to pH 7.4 at pCO2 = 40 mmHg. positive value = base excess negative value = base deficit (BD) Astrup determination is based on measurement of pH, pCO2, pO2 in blood It is measured by special electrodes in automatic apparatus. An arterial blood sample is used. Normal values of arterial blood: • pH = 7.35 – 7.45 • pCO2 = 4.8 – 5.8 kPa = 36 – 43.5 mmHg • pO2 = 9.8 – 14.2 kPa = 73.5 – 106.5 mmHg These values are measured directly. Concentration of HCO3- and BE are calculated from measured values by software in automatic apparatus. Astrup apparatus • Glass electrode – pH • Membrane electrode – pCO2 • Clark´s oxygen electrode – pO2 Anion gap (AG) AG represents the plasma anions which are not routinely measured (albumin, phosphates, sulphates, organic anions). AG is calculated as follows: AG = (Na+ + K+) – (HCO3- + Cl-) The sum of the concentrations of Na+ and K+ is greater than the sum of concentrations of HCO3- and Cl-. Difference is called as a anion gap. Normal values of AG: 16 – 20 mmol/L AG is calculated in case of metabolic acidosis. ABB disorders ACIDOSIS respiratory metabolic ALKALOSIS respiratory metabolic Compensation of ABB disorders • Metabolic disorder is compensated by respiration and conversely Correction of ABB disorders • Metabolic disorder is corrected by metabolic processes Respiratory acidosis (RAc) RAc is caused by hypoventilation (or breathing of CO2 containing air). Hypoventilation is associated with an impaired ability to eliminate CO2, whereby pCO2 increases and the accumulated CO2 reduces the arterial pH. Causes: airway obstruction, neuromuscular disorders, disorders of CNS, opiate poisoning Compensation: ↑ reabsorption of HCO3- is performed by kidneys (proximal tubule) Respiratory alkalosis (RAl) The hyperventilation is disproportionately high compared to the CO2 production, whereby the pCO2 falls and the pH increases Causes: CNS injury, salicylate poisoning, fever, … Other typical cases are the anxious patient during an attack of asthma or the hysterical hyperventilation in neurotic patients. Compensation: ↑ renal excretion of HCO3- → plasma pH decreases toward normal pH Metabolic acidosis (MAc) MAc is caused by accumulation of acids in ECF. ● negative BE ● Causes: • hypoxia is a lack of O2 in tissues → anaerobic glycolysis produces lactic acid → lactate acidosis • overproduction of ketone bodies → ketoacidosis (DM, starvation) • ingestion of methanol or ethylene glycol • diarrhoea Compensation: 1st step: buffering of excess of H+ by HCO32nd step: respiratory compensation by hyperventilation 3rd step: renal correction → ↑ excretion of H+ in urine Metabolic alkalosis (MAl) MAl is caused by a primary accumulation of bases in ECF. Both the [bicarbonate] and the [non-carbonic buffer base] are increased, so the BE is increased. Causes: • ingestion of alkaline drugs (e. g. NaHCO3) • prolonged vomiting → loss of H+ Compensation: 1st step: buffering of excess of HCO32nd step: respiratory compensation by hypoventilation → ↑ pCO2 in alveoli and arterial blood 3rd step: renal correction: ↑ excretion of HCO3- in urine Case report 1 A young man was injured in the chest from a car accident. Instrument ventilation was started. plasma HCO3- measured values 25 mmol/L Astrup pH pCO2 pO2 7.24 60 mmHg = 8 kPa 60 mmHg = 8 kPa Type of ABB disorder?? Solution of case report 1 Respiratory acidosis without compensation. Hypoventilation is a cause of ↑ pCO2 in arterial blood. Case report 2 A 45 year old man was admitted with a history of persistent vomiting. He had a long history of dyspepsia. Examination revealed dehydration and shallow respiration. plasma measured values K+ 2.8 mmol/L HCO345 mmol/L urea 34 mmol/L Astrup pH 7.56 pCO2 54 mmHg = 7.2 kPa Type of ABB disorder?? Solution of case report 2 Metabolic alkalosis is a result of persistent vomiting loss of H+ and dehydration. Small amount of urine (lower diuresis) is a cause of higher concentration of urea in blood. Respiratory compensation was started (hypoventilation) → ↑ pCO2. Lower K+ concentration indicates alkaleamia. Case report 3 A 23 year old mechanic was admitted to hospital 12 hours after drinking antifreeze. He was given 400 mmol of HCO3- with a little effect. Dialysis was started but he went to shock and died 12 hours after admission. plasma admission Na+ 137 mmol/L K+ 5.4 mmol/L Cl95 mmol/L HCO3- 4 mmol/L Glc 2.5 mmol/L Astrup pH 6.95 pCO2 15 mmHg dialysis 4 hours 145 mmol/L 4.9 mmol/L 87 mmol/L 5 mmol/L 7.05 16 mmHg Type of ABB disorder?? 7.29 25 mmHg = 3.33 kPa Solution of case report 3 Metabolic acidosis is due to antifreeze poisoning. Antifreeze contains ethylene glycol which is oxidized to oxalic acid in body. After 12 hours, the respiratory compensation was started → hyperventilation → ↓ pCO2. Cause of his death is a renal failure due to oxalates in kidneys. Case report 4 A young woman was admitted 8 hours after taking an overdose of aspirin. plasma HCO3Astrup pH pCO2 measured values 12 mmol/L 7.53 15 mmHg = 2 kPa Type of ABB disorder?? Solution of case report 4 Respiratory alkalosis is due to overdose of aspirin. pCO2 is decreased because patient has a hyperventilation. Renal compensation was started → excretion of HCO3-.