Inorganic Chemistry review sheet Exam #3 Ch. 9 Lewis acids (e
advertisement

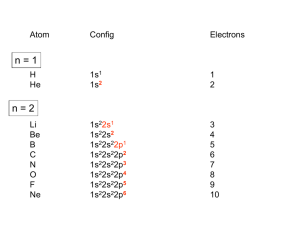
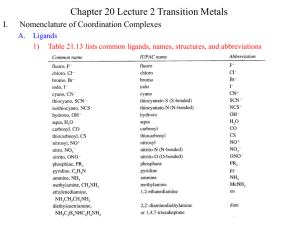
Inorganic Chemistry review sheet Exam #3 Ch. 9 Lewis acids (e– acceptor; TM ion) & bases (e– donor; ligand). Most RXNs can be labeled as Lewis A/B. Useful to recognize Lewis acids & bases in order to predict chemical reactivity & understand coordination chemistry. Ligand: molecule or ion that can bond (coordinate) to a metal ion through an e– pair. Driving force, K, to form a coordinate covalent bond, or A/B strength, depends on several factors: Strength of Lewis acid/base: 1. Electronic effects (χ of substituents & orbital overlap) 2. Steric effects (size) 3. Hybridization (% s-character; more s-character, more χ) 4. Polarizability (hard/soft acid/base) Hard/hard, soft/soft (Keq) (Pearson) On Periodic Table: soft on bottom left, hard on top right. Ch. 11 – 13 Most of inorganic chemistry: coordination and organometallic. Transition Metal Chemistry addresses their unique properties of structure, magnetism & color. Typically partially filled d-orbitals. (d e– in first row M2+ ions = Z – 20) Calculating # of d-electrons (remember, s e–s removed before d e–s). Father of Inorganic chemistry, Alfred Werner Coordination chemistry monodentate (bonds through one atom) bidentate (bonds through two atoms) chelating (claw) if the bidentate species binds to the same metal species Nomenclature 3. Metal a. b. Exs. c. Cationic or neutral complex: Metal name unchanged. Anionic complex: Latin form (usually like symbol) with –ate ending. cuprate ferrate aurate argenate Oxidation state: Roman numerals in parentheses. Stock notation. No space between metal and roman numerals! Putting it Together 4. Order a. cation, anion b. ligands 1st; attach directly to metal name c. abc order 5. a. b. c. 6. neutral ligand name in parentheses except for H2O, NH3, CO, NO short anions not in parentheses long anions (4 syllables) in parentheses # of each type of ligand indicated by prefixes. a. short names di, tri, tetra, penta b. long names bis, tris, tetrakis (when in doubt, use these) Note: the prefix is NOT included in the alphabetization! cis- and trans- indicate ligands on same side and across, respectively (for square planar and Oh). On an Oh complex, facial (fac-) and meridonal (mer-) indicate ligands on a triangular face and on the equator, respectively. Isomers. (Geometrical, linkage, conformational, optical) Can explain special properties of magnetism, spectroscopy (color) & reactivity (labile, inert). dorbitals are focus. VB Theory, MO Theory (seen) as well as: Crystal Field Theory (CFT): Assumes 100% ionic bonding. Works for many coordination compounds, even if not primarily ionic. Intuitive & easy. Ligand Field Theory: Starts with CFT, includes parameters that add covalency. d-orbitals (in 3-D space): d-orbitals are degenerate in a spherical field (no ligands present). Once ligands approach, Splitting of the d-orbitals: Oh __ __ eg Td __ __ __ t2 Use lower case when dealing with 1 e– systems. __ __ e __ __ __ t2g Crystal Field Stabilzation Energy (CFSE) for Oh (t2g = –4Dq, eg = +6 Dq) Crystal field splitting: 1. Charge on TM ion (larger charge, greater splitting) (HS vs. LS) 2. Size of the TM (radial distribution of d-orbitals increases going down the group) 3. Nature of the ligand, spectrochemical series: I– < Br– < OCrO32– chromate < Cl– SCN– < N3– < F– SSO32– thiosulfate urea (O) < OCO22– carbonate < OCO2R– carboxylate < ONO– OH– < OSO32– sulfate < ONO2– nitrate < O2CCO22– oxalate (bidentate) < H2O < NCS– < glycine EDTA4– < pyidine NH3 < en < SO32– < bipy < o-phen < NO2– < Cp < CN– (halogen-weak field, oxygen containing- medium field ligand, nitrogen and carbon-strong field ligand) 4. Geometry (Oh greater splitting than Td (see above)) Splitting of the d-obitals under the influence of different crystal fields: Tetragonal Distortion (Oh) (elongation or compression yield different splitting) Td can also undergo distortions Jahn Teller effect: “Any non-linear molecular system in a degenerate electronic state will be unstable and will undergo distortion to form a system of lower symmetry and lower energy thereby removing the degeneracy." Can influence symmetry (will see one point group via crystallography instead of another). Crystal Field Stabilization Energy (CFSE) When placing e–s into d-orbitals, sometimes there is a choice for high spin (HS) or low spin (LS); depends on Δo & E needed to pair 2 e–, pairing E (P). Ex. d4 In theory, could have HS & LS Td complexes. All known Td complexes are HS because t not very large. After d7, no longer a choice of HS or LS in Oh. Guoy balance- used to determine if TM is diamagnetic (all e–s paired up) or paramagnetic (at least one unpaired e–) ______ Calculate effective magnetic moment, μSO = √n(n+2) from attractive force. Compare μeff with spin only magnetic moment, μSO. μeff comes from the spin & orbital motion of an e–. Spin dominant for most compounds. Orbital contribution from asymmetry in orbital filling. Preferred geometry: a balance between CFSE, P, & sterics. Transition metal complexes usually highly colored. To explain, 1st look at MO theory of TM complexes. Difficult but more quantitatively accurate than CFT. A ligand is not a point charge; must have covalent character. e– transitions are cause of colors of TM complexes (spectroscopy): I. Charge Transfer, e– moving from metal to ligand or ligand to metal. II. Ligand to ligand (not a visible transition). III. Ligand Field Transitions (metal to metal, aka d to d transitions). Responsible for color of complexes. Oh weaker than Td. Visible color is compliment of color absorbed. (ROYGBIV) Peaks in spectra broadened due to ligands moving towards and away from TM. Term symbols (act like orbitals. Shorthand for energy and spin of a state). L = Σ ℓ, S = Σ ms (spin) Hole formalism (missing e– acts like a positron). d9 Oh is like d1 Td: (d8 and d2, d7 and d3 similarly related). Don’t need terms until > 1 e– (b/c of e–/e– repulsion). Find terms in a methodical way (make the table, generic example shown, actual table needed will vary depending on example): Ground state term is one with highest L and maximum multiplicity. Tanabe-Sugano diagrams (see link) (weak field (left) to strong field (right)). Using them to interpret spectra. Transition Selection Rules: 1. Laporte- g to g (u to u) forbidden. Vibronic coupling removes i. Td larger ε than Oh (as mentioned above). 2. Spin selection rule- Transition between different spin multiplicities forbidden. Partially relaxed with spin-orbit coupling. 3. Multiple electron transitions not favorable, but not forbidden. Nephelauxetic Effect (“cloud expansion”)