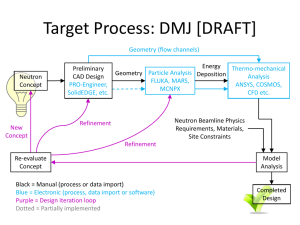
Neutron Activation Analysis

Neutron Activation Analysis
References:
Alfassi, Z.B., 1994, Determination of Trace Elements, (Rehovot: Balaban Publ.)
Alfassi, Z.B., 1994b, Chemical Analysis by Nuclear Methods, ( Chichester: Wiley)
Alfassi, Z.B., 1990, Activation Analysis, (Boca Raton: CRC Press), p. 161.
Balla, M., Keömley G., Molnár Zs., 1998,
Neutron Activation Analysis in
Vértes, A., Nagy S.,
Süvegh K., Nuclear Methods in Mineralogy and Geology (New York: Plenum), chapter 2, pp.115-143.
Handbook of Nuclear Chemistry
Contents
• Principle of activation analysis (AA)
• Different types of AA
• Neutron Activation Analysis (NAA)
- different types of NAA (prompt - delayed, instrumentalradiochemical)
- neutron sources used for NAA
- measuring equipment used for NAA
- quantification of NAA (absolute, relative and comparator techniques)
- properties of INAA (sensitivities, and non-destructive multielement character)
- radiochemical separations in NAA
• INAA - application examples
• RNAA – application examples
INCIDENT
NEUTRON
Principle of activation analysis
PROMPT
GAMMA RAYS
COMPOUND
NUCLIDE
DECAY
GAMMA RAYS
RADIOACTIVE
NUCLEUS
X
CHARGED
PARTICLE
PHOTON
TARGET
NUCLIDE
STABLE
NUCLEUS
Elemental – multi-elemental – trace elemental analysis
Steps of analysis: sample preparation (homogenization, weighing) optional: pre-irradiation chemistry irradiation cooling (different times) optional: post-irradiation chemistry measurement by gamma spectrometry evaluation
History
• Hevesy and Levi 1936: principle of NAA
• Neutron sources became available in the fifties
• Low resolution detectors (proportional counters, NaI scintillator)
• High resolution semiconductor detectors
• Alternative non-nuclear methods (AAS,
ICP-OES, ICP-MS)
Various types of AA
1. Charged particle activation analysis
2. Photon activation analysis
3. Neutron activation analysis (NAA)
1. Thermal neutron activation analysis
2. Epihermal neutron activation analysis (ENAA)
3. Fast neutron activation analysis (FNAA)
4. Neutron capture prompt gamma activation analysis (PGAA)
Neutron sources used for NAA
Isotopic neutron sources:
-emitter
227 Ac
226 Ra
239 Pu
210 Po
Half life
22 y
1620 y
2.4x10
4 y
138 d
Neutrons
s -1 Ci -1 emitted
1.5x10
7
1.3x10
7
1.4x10
7
2.5x10
6 average
neutron energy [MeV]
4
3.6
4.5
4.3
Spontaneous fission of actinides:
252 Cf (half life 2.6y): 3.76 neutrons of 1.5 MeV per event
1mg 252 Cf emits 2.28x10
9 neutrons/sec
Neutron generators: deuterons accelerated by 200 kV: 3 H(d,n) 4 He monoenergetic neutrons: 14 MeV (fast n reactions: (n,p), (n, α), (n,2n)) neutron yields: 10 11 neutrons/s/ mA, neutron flux: 10 9 neutrons/cm2/s
Research reactors
Research reactors as neutron sources: thermal power: 100 kW-10 MW thermal neutron flux: 10 12 -10 14 neutrons cm-2 s-1 thermal
<0.05 eV epithermal +resonance
0.1eV<E<1 eV
1eV<E<1 keV mean:0.04 eV
2200 m/s
(n, γ) cold neutron beam
(n, γ) fast neutrons
0.5 Mev<E
(n,p),(n, α),(n,2n)
Measuring systems used for NAA
Gamma spectrometers: scintillation detector
Ge(Li) detector
HP Ge detector
Quantification
A
∞
=N R
N
0 s
( E )
( E ) dE
N: number of interacting isotopes s
(E): cross-section [cm2] at neutron energy of E [eV] f
(E): neutron flux per unit of energy interval [1/cm2/s/eV]
R: reaction rate
In reactors the integral is replaced:
R
th s th
epi
I
0 s th
: conventional thermal neutron flux [in cm2] f th f e
: effective thermal neutron cross-section [in cm2]
: conventional epithermal neutron flux [in cm-2 s-1 eV-1]
I o
: resonance integral cross section (in epithermal region), for 1/E epithermal spectrum [in cm2]
A = (
th
s th
e
I o
) m
f i
N
Av
A rel
N
Av
: Avogadro number fi : isotopic abundance m : the mass of the irradiated element
A rel ti :
: atomic mass of target element time of irradiation; td : time of decay; l
: decay constant
S: saturation factor: S=1-e l ti
D: decay factor: D=e l td
S
D
Activity is determined from measurements:
N
P
A f
t m
N p
: net peak area,
: detection efficiency, f
: gamma abundance, t m
: measuring time
Combining the last 2 equations, the mass of the unknown element can be calculated: m
N
Av f i f
N p
M
(
th s th
e
I
0
) SDt m
Concentration c is determined from measured
„m” and the volume/mass (V/G) of the sample: c=m/V and c=m/G, respectively .
Standardization
• Absolute method
Based on the expression of
„m”
Parameters to be measured:
Np, tm, ti, td
Parameters to be determined by calibration:
ε, φ th
, φ e
Parameters derived from tables (nuclear+additional):
σ th
, I
0
, f
γ
, f i
, λ, N
Av
, M
• Relative method
Each element is measured against an „element standard” irradiated together with the sample
I m
I sp
I
N p
SDt m
I sp
N sp
S sp
D sp t m , sp m sp
Parameters not used:
ε, φ th
, φ e
, σ th
, I
0
, f
γ
, f i
, N
Av
, M
• Comparator method: „k” method
All elements are measured related to a single element, the comparator.
Calibration phase : k factors are determined for each element compared to the comparator element
(irradiation together)
* refers to comparator element
‘ refers to analyte in calibration procedure
Measurement phase:
Sample is irradiated together with the single comparator, sample and comparator are measured
** refers to comparator element during measurement phase k
I sp
*
I sp
N p '
S
'
D
' t m ' m
'
N p *
S
*
D
* t m * m
* m
I
I
* * k sp
N p
SDt m
N p * * k
S
* *
D
* * t m * * m
* *
k factors are constant under constant irradiation and measurement conditions
(including the same geometry): k
M
*
Mf
* f
f i * f i
*
(
th s
(
th s th th *
e
e
I
0
)
I
0 *
)
M
*
Mf
* f
f i
s f i *
* s th th *
(
th
(
th
/
e
/
e
I
0
I
0 *
/ s
/ s th
) th *
)
• the k
0 method
A pure nuclear constant k
0 was derived from k factor by deCorte, that is independent of measuring and irradiation conditions: k
0
M
* f
M f
* f i s f i * s
*
This value was measured and checked.
Knowing k
0 the standardization procure is simplified or omitted. „k” can be calculated
.
K
0 values were experimentally determined/checked according to the following equation: k
0
I sp
I sp *
*
(
(
th th
/
/
e e
I
I
0 *
0
/ s
/ s th *
) th
)
Properties of INAA
Advantages
• Sensitive, trace elements are determined
• Multi-element method
• Matrix dependence is often small
• Non-destructive
Disadvantages
• Neutron source and gamma spectrometer are needed
Expensive and „nuclear” method
Application of INAA
Geological samples:
• NAA at INT-TU Budapest
- 1 minute irradiation,15 min cooling times: (28Al decays) Ti, V, (Cu),
Mn, Cl, Dy and Ca are determined.
- 8 hour irradiation in a thermal channel of the reactor measurment twice: one week, one month
- usually 25-30 elements can be determined
• Epithermal NAA
- gross activity due to 24Na, 56Mn, 46Sc, 28Al: low I o
/ σ th
- analytes (Rb, Sr, Ba, Ga, As, Mo, Ag, In, Sn, Sb, Sm, Tb, Ho, Ta,
W, Au, Th, U): high I o
/ σ th
- epithermal AA in Cd wrapping → high sensitivity.
• Disturbing nuclear reactions
- The same radionuclide is produced from two different elements: e.g. 28Al : 27Al(n, γ)28Al 28Si(n,p)28Al.
thermal n fast n
- samples can be activated twice, with and without cadmium filter, in order to determine both Al and Si
• Studies on lanthanides to derive concentrations relative to standard condrites volcanic activities
Biological samples
- Analysis of Na, K, Al, Se in brain samples to study deseases e.g.
Alzheimer
Archaeological samples
Provenance studies on Roman ceramics
Provenance studies on the jars storing the Dead Sea Scrolls
Gold in fibres of the royal gown
See the home page for details!
Types of radiochemical NAA
• Post-irradiation chemistry ( RNAA ) no contamination hazard addition of carriers – no radiocolloids yield determination shielded, remote-controled devices separations: matrix separation group separation single element separation
• Pre-irradiation chemistry ( PC NAA ): pre-concentration contamination hazard
• Pre- and post-irradiation chemistry ( PC RNAA ) extremely high sensitivites e.g. 129I determination in environment
• Chemical AA ( Ch NAA ): separation for speciation purposes pre-irradiation (irradiation may change the chemical conditions)
Chemistry is typically simple!
Application of RNAA in material sciences
Separation of the matrix
• Analysis of impurities in high purity Al
27Al(n,α)24Na
Na separation by HAP
• Analysis of Ni based alloys separation of Ni by DMG
• Analysis of Mo/W coumpounds
Mo and W separation by anion exchangers
Separation of single elements
• Si analysis in Mo: 32Si is short-lived, can be counted by beta detector, Si is separated
• P analysis in semiconductors: 32P is pure beta emitter separation with AMP
Application of RNAA for the analysis of biological, environmental and geological samples
• Matrix removal: matrices are: Na, K, P, Br, Cl removal: HAP TiO2 Al2O3 volatilization
• Single element separations:
- Se:toxic/micro-nutrient separation by extraction or precipitation of elemental Se with ascorbic acid
- I: essentiel element separation: I2 extraction + AgI
- Hg: toxic element separation by volatilization or extraction + precipitation as HgI2 or HgS or Hg
- Sr: major interest as natural carrier for 90Sr separation by co-precipitation with Ca
- Th and U: radioactive elements
Th:separation of 233Pa by co-precipitation with MnO2 and BaSO4
Th:PC RNAA of Th: pre-conc of Th by ion exchange separation of 233Pa by ion exchange or extraction
U, Th: separation of 239Np and 233Pa by TBP
• Single group separations
Pt group elements:Ru, Rh, Pd, Os, Ir, Pt. history of rocks environmental concern: catalysts in cars, medicines
- RNAA: OsO4 + RuO4 /CCl4 extraction, anion exchange of chlorides
- PC NAA: fire assay/NiS preconcentration
Rare earth elements (REE):
- co-precipitation with ferric hydroxide
- others: ion exchange, extraction
• Several elements and various groups separations
„historic significance
Pietra method: 50 elements in biological materials separation by all types of analytical methods (volatilization, ion exchange, sorption…)
Application of RNAA for the determination of radionuclides
• 129 I
PC RNAA: volatilization, extraction of I
2
, precipitation of PdI
2
• 237 Np
• 99 Tc
Application of NAA for speciation studies ChNAA
• Non-protein bound Al or protein bound Al in urine: role in osteomalacia separation by cation exchange
• Iodine speciation in sea water separation by anion exchange
Nem nukleáris elemanalitikai módszerek
Tömegspektrometria: SS MS
TI MS
ICP MS
GD MS
Röntgenfluoreszcencia: WD XRF
ED XRF
TR XRF
SR XRF
PIXE
EPMA
Optikai módszerek: abszorpciós: emissziós:
Elektrokémiai módszerek: Voltammetria
Coulombmetria
Polarográfia
AAS (láng, lámpa)
OES (=AES)
ICP OES
Elemanalitikai módszerek
érzékenysége
összehasonlítás