(banana).
advertisement

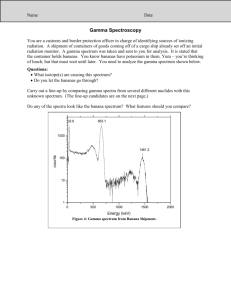
What nuclides made that electromagnetic radiation? How nuclear scientists identify nuclides in a sample Accelerating charged particles produce EM radiation pattern characteristic of charged particle(s) producing it White light from incandescent lightbulb produced all colors of light http://www.dnr.sc.gov/ael/personals/pjpb/lecture/spectrum.gif Light from gas tube (neon, mercury, etc) produces only some colors http://fuse.pha.jhu.edu/~wpb/spectroscopy/basics.html Characteristic Atomic Spectra Atomic Neon (electronic) transitions produce “line” http://fuse.pha.jhu.edu/~wpb/spectroscopy/basics.html spectra All atoms have Argon unique spectra Spectral analysis identifies atoms in the sample http://www.gc.maricopa.edu/earthsci/imagearchive/Argon%20Spec%20sm.jpg http://www.aanda.org/articles/aa/full/2008/03/aa5969-06/img139.gif. Atomic VS Nuclear Spectra: 41Ar Atomic spectrum electron emits EM radiation when making transition to lower energy level uv, visible and x-ray radiation possible Nuclear spectrum nuclei emit EM radiation when making transitions to lower energy state X-ray and gamma ray radiation possible Identifying Isotopes You are a customs and border protection officer in charge of identifying sources of ionizing radiation. A shipment of containers of goods coming off of a cargo ship already set off an initial radiation monitor. A g-spectrum was taken and sent to you for analysis. It is stated that the container holds bananas. You know bananas have potassium in them. Banana Crate Spectrum Questions: •What isotope(s) are causing this spectrum? •Do you let the bananas go through? The “photo lineup” 137Cs Banana Crate Spectrum 28Al 38K 40K U-ore Spectrum library for MOST nuclides is available at Idaho National Laboratory site: http://www.inl.gov/gammaray/catalogs/nai/catalog_nai.shtml What are these nuclides? Potassium-40 (40K) is the second most abundant radioactive substance on Earth. 40K has a signature peak in its g spectrum for g-rays with kinetic energy of 1460 keV (or a wavelength of 849 fm = 849 x 10-15 m). Cesium-137 is produced in nuclear fission. Small amounts of this are found in nature, however human activities (nuclear weapons detonations and the fallout from Chernobyl and probably the Fukushima Daiichi accidents) released 137Cs into the environment. This nuclide has a signature gray peak at 661.2 keV. Could these nuclides be in bananas? Would these nuclides be in bananas? Bananas are naturally high in Potassium, so having some 40K is normal. Some plants are able to uptake radioactive isotopes and concentrate them in their fruit, e.g., berries, mushrooms, and grasses. The bananas have done this with the Cs. However, someone could be illegally transporting 137Cs in the container. Further study may be needed!