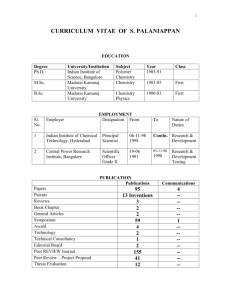
Presented By: Deepshikha
Amity University
Noida
Objective
Optimization of process parameters to find out
the best nanostructured conducting polyaniline in
terms of conductivity and size.
Application
of
nanostructured
polyaniline to biosensors.
conducting
Conducting Polymer
A conductive polymer is an organic polymer
semiconductor. They provide pathways for
electronic conduction by doping. Common classes
of organic conductive polymers include
Poly(acetylene)s,
Poly(pyrrole)s,
Poly
(thiophene)s, Poly(aniline)s etc.
Characteristics of Nanostructured
materials
Nanostructures are inorganic, organic materials with one
dimension down to the nanometer range i.e. within 1-100
nm.
Unique aspect of nanomaterials is the vastly increase
ratio of surface area to volume
Dramatic changes in properties (electrical, thermal,
mechanical, electronic, optical) in nano scale dimension
from their bulk material.
Biosensor
Bio sensor is an analytical device which converts
a biological response into readable signal .
Bio sensor comprises of three components:
bioreceptor, transducer and detector.
Advg of Nanostructured Conducting
Polymers (NSCP) for Biosensor appl
High Surface Area
Unique optical, electronic and magnectic properties
Bio-compatibility
Dimensional compatibility with biomolecules.
Film forming ability.
Flexibility and processibility.
Size, stability, morphology , conductivity and porosity of
nanostructured conducting polymers(NSCP) can be modified
by changing reaction conditions.
Unique π electron uni-dimensional conductivity- enhancing the
electron transfer rate(ETR) , lowering the detection potential
and enhancing the stability of the enzyme .
Electrochemical switching depending upon pH and state of
oxidation.
Polymerisation procedure
Aniline was dissolved into SDA solution
Temperature was maintained at 00 to 50C
Oxidizing agent, (NH4)2S2O8, in ice-cold water was
added
Polymerisation was allowed to proceed for 3 to 4 h
Mixture was allowed to age under static condition for 1-3
days for complete polymerization.
Effect of concentration of oxidizing
agent on the properties of NSPANI
500
3.5
450
400
Absorbance
3.0
2.0
800
PANI2
1.5
803nm
1.0
350
900
806nm
2.5
300
600
500
400
300
200
0
0.5
0.0
400
700
100
PANI1
PANI1
600
800
PANI2
PANI3
PANI4
1000
Wavelength(nm)
1.1
250
Polydispersity Index(PDI)
Resistance(Kilo ohms)
size distribution and the
highest conductivity
are
obtained when molar
concentration of
oxidizing agent is in the
range of 0.01-0.03M.
789nm
778nm
PANI3
PANI4
4.0
Z-Average(d-nm)
Smallest size, uniform
200
150
100
1.0
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
PANI1
PANI2
PANI3
PANI4
50
0
PANI1
PANI2
PANI3
PANI4
Effect of concentration of monomer on
the properties of NSPANI
790nm
PANI8
806nm
45
40
Absorbance
2.5
2.0
PANI2
1.5
1.0
240
220
Z-Average(d-nm)
3.0
50
200
180
160
140
120
0.5
100
35
0.0
400
600
800
1000
PANI2
Wavelength(nm)
30
25
20
15
0.62
Polydispersity Index(PDI)
Resistance(Kilo ohms)
Smallest size, uniform
size distribution and the
highest conductivity are
obtained when monomer
concentration is in the
range of 0.01-0.03M.
0.60
0.58
0.56
0.54
0.52
0.50
0.48
0.46
0.44
0.42
PANI2
PANI8
10
PANI2
PANI8
PANI8
Effect of concentration of structure
directing agent on the properties of
NSPANI
3.0
2.5
2.0
1.5
1.0
0.5
240
220
200
180
160
140
120
100
0.0
400
600
800
1000
PANI5
PANI2
PANI6
PANI7
Wavelength(nm)
0.65
Polydispersity Index(PDI)
Absorbance
Resistance (Kilo ohms)
Smallest size, uniform
size distribution and the
highest conductivity are
obtained when SDS
concentration is in the
range of 0.06-0.08M.
40
38
36
34
32
30
28
26
24
22
20
18
16
14
12
10
8
3.5
Z-Average(d-nm)
771nm
795nm
PANI5
PANI7
801nm
806nm
PANI2
PANI6
4.0
0.60
0.55
0.50
0.45
0.40
PANI5
PANI5
PANI2
PANI6
PANI2
PANI7
PANI6
PANI7
Effect of size of soft microreactor on size
of polymer nanoparticles
240
Avg. particle size(nm)
The size of PANI
nanoparticles decreased
with increasing the
surfactant concentration.
220
200
180
160
140
120
100
0.02
0.03
0.04
0.05
0.06
0.07
Conc. of surfactant(m/l)
0.08
Optimum process conditions for
nanostructured conducting polyaniline
Monomer: 0.01-0.03M
Structure directing agent: 0.06-0.08M
Oxidant: 0.01-0.03M
Temperature: 00- 50 C
Conditioning time: 1-3 days
Cyclic voltammetry was the method used
for electrodeposition of the
NSPANI
emeraldine salt (ES) onto ITO from the
aqueous dispersion of nanoparticles .
Main peaks A and B corresponding to the
transformation of leucoemeraldine base
(LB) to ES and ES to pernigraniline salt
(PS), respectively.
On the reverse scan, peaks B’ and A’
correspond to the conversion of PS to ES
and ES to LB, respectively.
Small redox peak around +350 mV
(C and C’) is associated with the formation
of p-benzoquinone and hydroquinone as a
side product.
Current(micro amperes)
Cyclic Voltammetric studies of best
nanostructured conducting polyaniline
B
0.00010
0.00005
C
A
0.00000
B'
-0.00005
C'
A'
-0.00010
-0.4 -0.2
0.0
0.2
0.4
0.6
Potential(V)
0.8
1.0
1.2
Application of nanostructured conducting
polyaniline to biosensors
Characterization of bioelectrode
Difference between the anodic and
cathodic peak potential is reduced on
deposition of NSPANI onto ITO
This indicates the reversibility and
electrocatalytic activity of the electrode
which may facilitate the electron
conduction pathway between the
enzyme and electrode.
Current(Amperes)
Marked decrease in the anodic and cathodic
peak current upon immobilization of
enzyme on NSPANI film which indicates
that enzyme has immobilised on NSPANI film
a
0.00020
b
0.00015
0.00010
c
0.00005
0.00000
-0.00005
-0.00010
-0.00015
-0.4 -0.2 0.0
0.2
0.4
0.6
Potential(V)
0.8
1.0
1.2
Pictorial representation of synthesis of NSPANI,
Immobilization of enzyme and biochemical reaction at
Enzyme/NSPANI/ITO bioelectrode
ITO Electrode
Film formation of NSPANI on ITO
Polyaniline nanoparticles (NSPANI)
Glutaraldehyde Enzyme
Polymerization of aniline in the
OHC-(CH2)3-CHO
presence of soft microreactor
0.60
b
0.50
Absorbance
CONH
GoX/NSPANI
0.55
Glucose+O2
0.40
CONH
Gluconic acid+H2O2
H2O2+O-anisidine
(Reduced)
0.45
CONH
O-anisidine
(Oxidised)
0.35
0.30
0.25
a
0.20
0
10
20
30
40
Immobilization of enzyme
50
Conc(mM/l)
on NSPANI film
Photometric response study for
the detection of analyte
Sodium dodecyl sulphate
Aniline
Enzyme
Photometric response studies of
glucose biosensor
GOx/NSPANI
Glucose + O2
Gluconic acid + H2O2
H2O2 + O-anisidine (red)
2 H2O + O-anisidine
(oxidized)
0.60
0.55
b
0.50
Absorbance
The value of absorbance resulting from the
oxidized form of dye was found to be increasing
linearly in the range of 5 mM/l to 40 mM/l for
GoX/NSPANI where as bulk PANI exhibits
linearity between 5-20mM.
0.45
0.40
0.35
0.30
0.25
a
0.20
0
10
20
30
Conc(mM/l)
40
50
Optical characteristics of various
glucose biosensors
Name of the Km(mM)
Linearity(m
Response
Lower
bioelectrode
M/l)
time(s)
detection
limit(mM/l)
GoX/NSPANI
0.28
5to40
30
0.1
GoX/bulk
21.0
5to 20
90
1
PANI
Effect of pH on GoX/NSPANI/ITO
bioelectrode
The higher value of the absorbance
At this pH, biomolecules retain their
natural structures and do not get
denatured.
0.76
Absorbance
obtained at pH 7.0 indicates
GoX/NSPANI/ITO bioelectrode is the
most active at pH 7.0.
0.78
0.74
0.72
0.70
0.68
0.66
5.8 6.0 6.2 6.4 6.6 6.8 7.0 7.2 7.4 7.6 7.8 8.0
pH
Effect of interference on
GoX/NSPANI/ITO bioelectrode
The effect of interferents such as
The results indicate the negligible
effect of these interferants on the
photometric response of
GoX/NSPANI/ITO electrode.
Absorbance
uric acid(UA), sodium ascorbate(SA)
on the glucose measurement has
been studied.
0.8
0.6
0.4
0.2
0.0
Glu
Glu/UA
Glu/SA
Storage Stability
GoX /NSPANI/ITO based optical biosensor
retains its 90% activity after 15 days.
The loss in the activity of biosensor is not due to
the denaturation of enzyme but it is due to the
poor adhesion of cast NSPANI film on the ITO
electrode.
Electrochemical response studies
of glucose bioelectrode
Anodic current increases with
Current(Amperes)
increased concentration of
glucose .
c
0.00015
b
0.00010
a
0.00005
0.00000
-0.00005
-0.00010
-0.4 -0.2
0.0
0.2
0.4
0.6
Potential(V)
0.8
1.0
1.2
Photometric response studies of
H2O2 biosensor
b
1.0
0.9
0.8
Absorbance
The value of absorbance resulting from
the oxidized form of dye has been found
to be increasing linearly in the range of
1 mM/l to 50 mM/l for HRP/NSPANI
where as the bulk PANI, exhibits
linearity between 10 mM/L to 30 mM/L
HRP/NSPANI
H2O2 + O-anisidine (red)
2 H2O + O-anisidine
(oxidized)
(Orange-red colour)
0.7
0.6
0.5
0.4
0.3
0.2
a
0.1
0.0
0
10
20
30
Conc(mM/l)
40
50
Optical characteristics of various
H2O2 biosensors
Name of the Km(mM)
Linearity(m Standard
samples
M/l)
HRP/bulk
Regression
Deviation
Coeficient
21.0
10 to 30
0.37
0.99
1.06
1 to 50
0.03
0.99
PANI
HRP/NSPANI
Storage stability
The stability of HRP/ NSPANI films along with bulk
PANI have been found to be poor which can be
explained by the degradation of PANI film by the
oxidation reaction of H2O2.
Conclusion
It is required to maintain a very specific concentration of monomer,
oxidant,SDA as well as dopant in order to achieve the best NSPANI
with respect to size , conductivity as well as electrocatalytic
properties.
With slight variation from the optimum condition of polymerization
causes a drastic change in the electrochemical properties.
This biosensor format has shown wide linearity range(1mM-50mM),
fast response time (30 s)(photometric), negligible interferent
effect(0.1%) and low Km value(0.28)
Further studies on overall electrochemical response and stability of
the sensor is needed to be carried out.
References
Vaseashta A & Malinovska D D, Nanostructured and nanoscale devices,
sensors and detectors,Science and Technology of Advanced Materials,6
(2005) 312
Dhand C, Singh S P, Arya S K, Datta M & Malhotra B D, Cholesterol
biosensor based on electrophoretically deposited conducting polymer film
derived from nano-structured polyaniline colloidal suspension. Anal. Chim.
Acta, 602 (2007) 244.
Solanki P R, Kaushik A, Ansari A A, Tiwari A & Malhotra B D, Multi-walled
carbon nanotubes/sol–gel-derived silica/chitosan nanobiocomposite for total
cholesterol sensor, Sensors and Actuators B ,xxx (2009) xxx.
Dhand C, Arya S K, Datta M & Malhotra B D, Polyaniline–carbon nanotube
composite film for cholesterol biosensor, Analytical Biochemistry 383 (2008)
194.
Langer J J, Filipiak M , Ke_cin´ska J, Jasnowska J ,Włodarczak J K &
Buładowski B , Polyaniline biosensor for choline determination , Surface
Science ,573 (2004) 140.
Nandi M A, Gangopadhyay R B & Bhaumik A, Mesoporous polyaniline
having high conductivity at room temperature, Microporous and Mesoporous
Materials, 109 (2007) 239.
References contd.
Mazur M, Tagowska M, Pałys B & Jackowska K, Template synthesis of
polyaniline and poly(2-methoxyaniline) nanotubes: comparison of the
formation mechanisms, Electrochemistry Communications, 5 (2003) 403.
Huang J & Kaner R B, A General Chemical Route to Polyaniline Nanofibers,
J. Am.Chem. Soc, 126 (2004) 851.
(a) Liang L, Liu J, Windisch C F J, Exarhos G J & Angew Y L , Direct
Assembly of Large Arraya of Oriented Conducting Polymer Nanowires,
Chem. Int. Ed, 41 (2002) 3665.
(b) Sawall D D, Villahermosa R M, Lipeles R A & Hopkins A R, Interfacial
Polymerization of Polyaniline Nanofibers Grafted to Au Surfaces, Mater, 16
(2004) 1606.
Kuwabata S, Fukuzaki R , Nishizawa R , Martin C R & Yoneyama H ,
Electrochemical formation of a polyaniline-analogue monolayer on a gold
electrode,Langmuir 15 (1999) 6807.
Martin C R, Template Synthesis of Electronically Conductive Polymer
Nanostructures ,Acc. Chem. Res. 28 (1995) 61
References contd.
(a) Stejskal J , Kratochvil P, Armes S P, Lascelles S F, Riede A, Helmstedt
M, Prokes J & Krivka I, Polyaniline Dispersions. 6. Stabilization by
Colloidal Silica Particles, Macromolecules, 29 (1996) 6814.
(b) Gangopadhyay R, De A & Ghosh G, Polyaniline-poly(vinyl alcohol)
conducting composite: material with easy processability and novel
application potential, Synth. Metals.123 (2001) 21.
Kresge C T, Roth W J L , Vartuli J C & Beck J S, Ordered Mesoporous
Molecular Sieves Synthesized by a Liquid-Crystal Template
Mechanism,Nature,359 (1992) 710.
Che S, Bennett A E G, Yokoi T, Sakamoto K, Kunieda H , Terasaki O &
Tatsumi T, A novel anionic surfactant templating route for synthesizing
mesoporous silica with unique structure, Nature Mater, 2 (2003) 801
M. G. Han, S. K. Cho, S. G. Oh, S. S. Im,Preparation and characterization of
polyaniline nanoparticles synthesized from DBSA micellar solution. Synth.
Met. 126(2002), 53.
G. G. Wallace, G. M. Spinks, L. A. P. Kane-Maguire, P. R. Teasdale, in
Conductive Electroactive Polymers (2nd. ed), CRC Press, London 2003, p.
237.
References contd.
Moel K D , Ekenstein G O R A V, Nijland H, Polushkin E & Brinke G T,
Polymeric Nanofibers Prepared from Self-Organized Supramolecules,
Chem. Mater,13 (2001) 4580.
Carswell A D W, O’Rear E A & Grady B P,. Adsorbed Surfactants as
Templates for the Synthesis of Morphologically Controlled Polyaniline and
Polypyrrole Nanostructures on Flat Surfaces: From Spheres to Wires to Flat
Films, J. Am. Chem. Soc.,125 (2003) 14793.
Zhang X & Manohar S K , Polyaniline Nanofibers: Chemical Synthesis
Using Surfactants, Chem. Commun, 20 (2004) 2360.
Moulton S E, Innis P C, Kane-Maguire L A P, Ngamna O & Wallace G
G, Polymerisation and characterisation of conducting polyaniline
nanoparticle dispersions, Current Applied Physics 4 (2004) 402.