eScholarShare - Drake University
advertisement

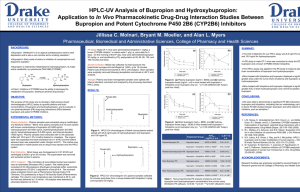
Carbonyl Reduction of Bupropion in Human Liver Cytosol Jillissa C. Molnari and Alan L. Myers Department of Pharmaceutical, Biomedical and Administrative Sciences, College of Pharmacy and Health Sciences, Des Moines, IA RESULTS: BACKGROUND: Human carbonyl reductases (CBRs) are primarily cytosolic enzymes that catalyze the reduction of many clinically relevant drugs.1 SUMMARY: Bupropion is reduced to TBUP and to a lesser extent EBUP in h liver cytosol (Figure 1). (a) Pharmacogenetic variations in CBRs have led to serious adverse consequences with drugs metabolized by CBRs, such as anthracycline chemotherapeutics.2 Bupropion reduction was linear with increases in cytosolic prote concentration and incubation time, and was nullified in the presen heat-inactivated cytosol (data not shown). Phenotypic probe substrates (e.g. dextromethorphan) are used to identify pharmacogenetic variations in drug metabolizing enzymes (e.g. CYP2D6).3 Metabolism of bupropion to EBUP and TBUP is NADPH depen (Figure 2). Apparent Michaelis-Menten constants (Km) for EBUP and TBUP formation were 150 µM and 100 µM, respectively. The maximal v (Vmax) for EBUP and TBUP formation were 43 pmol/min/mg and 2 pmol/min/mg, respectively. However, such substrates for CBRs are poorly studied. Bupropion may be a novel probe substrate since it is reduced to two major metabolites: erythrohydrobupropion (EBUP) and H H threohydrobupropion (TBUP): CH EBUP When tested at their reported literature IC50 values1, dicumarol, flufenamic acid and 4-methylpyrazole did not significantly inhibit metabolite formation (Figure 3). 3 OH N H C (C H 3 ) 3 (b) O H Cl CH3 Menadione (100 µM) significantly inhibited EBUP and TBUP fo (p< 0.001) (Figure 3). OH N H C (C H 3 ) 3 H CH3 Cl H Figure 3: Percent inhibition (of control) of EBUP and TBUP formation (by selective CBR inhibitors: dicumarol (0.2 µM), F.A. (5 µM), 4-MP (400 µM), and menadione (100 µM). F.A. (flufenamic acid); 4-MP (4methylpyrazole); ## p<0.001 TBUP N H C (C H 3 ) 3 Bupropion Cl CONCLUSIONS: Although no studies have been published to date, bupropion reduction is likely catalyzed by human cytosolic CBRs in the liver. Ketone reduction of bupropion in human liver cytosol appears t preferentially catalyzed by carbonyl reductase (CR), as evidence selective inhibition by menadione. OBJECTIVE: The main purpose of this study was to identify a major human CBR catalyzing bupropion reduction in human liver cytosol. EXPERIMENTAL METHODS: In Vitro Incubations: General incubation conditions were adapted from a literature method.4 Briefly, bupropion (100-700 µM), NADPH regeneration system (BD Gentest), potassium phosphate buffer (pH 7.4) and human liver cytosol (200 µg) were combined and incubated at 37ºC in a shaking water bath incubator. Control reactions contained heatinactivated cytosol. Incubation conditions were optimized for the bupropion concentration, cytosolic protein and incubation time that resulted in linear EBUP and TBUP formation. NADPH dependence was tested in the presence of NADPH regeneration system, NADPH, NADH or no co-factor. All reactions were terminated with a solution of 90% acetonitrile/0.10 M HCl, centrifuged and analyzed by HPLC for the formation of EBUP and TBUP. In Vitro Inhibition Studies: Dicumarol (0.2 µM), flufenamic acid (5 µM), 4-methylpyrazole (400 µM), and menadione (100 µM), specific inhibitors of quinone reductase, aldo-keto reductase, alcohol dehydrogenase, and carbonyl reductase enzymes, respectively1, were tested for inhibition of bupropion reduction. Data Analysis: Enzyme kinetic data was fitted to a Michaelis-Menten model using Enzyme Kinetics v1.1 software (SPSS, Inc.). IC50 plots and data analysis were performed by GraphPad Prism Software (v 5.0). IC50 values for inhibition of this reaction by menadione were 27 88 µM, respectively (Figure 4). The extent of this reaction in human liver microsomes (which e the 11-HSD enzyme) will be studied in the near future. Figure 1: Bupropion concentration (100 µM to 700 µM) vs. Rate of metabolite (a) EBUP and (b) TBUP formation (pmol/min/mg). To conclude, bupropion shows promise as a novel in vivo probe substrate for identifying pharmacogenetic variations in CR, which ultimately benefit patients prescribed drugs that are substrates fo REFERENCES: Figure 2: Reaction Co-factor vs. Rate of EBUP and TBUP formation (pmol/min/mg) plot showing the NADPH dependence of the reaction. R.S. (NADPH regeneration system, BD Gentest) Figure 4: Percent inhibition (of control) of EBUP and TBUP formation by the selective carbonyl reductase (CR) inhibitor menadione (50 µM to 400 µM). IC50 values for inhibition of EBUP and TBUP formation were calculated as 27 µM and 88 µM, respectively. 1) Rosemond MJC and Walsh JS. Human carbonyl reduction pa and a strategy for their study in vitro. Drug Metab Rev 2004; 335-361. 2) Lakhman SS, Ghosh D and Blanco JG. Functional significanc natural allelic variant of human carbonyl reductase 3 (CBR3). Metab Dispos 2005; 33(2): 254-257. 3) Wojtczak A, Rychlik-Sych M, Krochmalska-Ulacha E, Skretko CYP2D6 phenotyping with dextromethorphan. Pharmacol Rep 59(6): 734-738. 4) Porter SJ, Somogyi AA and White JM. Kinetics and inhibition formation of 6-naltrexol from naltrexone in human liver cytos Clin Pharmacol 2000; 50(5): 465-471. ACKNOWLEDGEMENTS: Research funding was graciously provided by the Drake Universi College of Pharmacy and Health Sciences. We thank Dr. Brian G (Drake University) for assisting in the IC50 analyses.