First Course in Organic Chemistry
2010 - 2011
Introduction to Organic Chemistry
By Dr. Nabila Aljaber
08/04/2015
1
WARNING!
•This document contains visual aids for lectures
•It does not contain lecture notes
•It does not contain actual lectures
•Failure to attend lectures can harm your
performance in module assessment
08/04/2015
2
Printing out
handouts of
PowerPoint
documents
Click on ‘OK’
Set ‘Slides per
page’ to ‘3’
(recommended
to facilitate
taking of notes),
‘4’ or ‘6’
08/04/2015
From ‘File’
menu,
select
‘Print’
Set ‘Print
range’ to
‘All’; set
‘Print
what:’ to
‘Handouts’
3
What is Organic Chemistry?
It is defined as the study of hydrocarbons
(compounds of hydrogen and carbon) and
their derivatives
7 million Organic Compounds
1.5 million Inorganic Compounds
Animal and plant matter, Foods,
Pharmaceuticals, Cosmetics, Fertilizers,
Plastics, Petrochemicals, Clothing
08/04/2015
4
Periodic Table
08/04/2015
5
Carbon
Why is it the element of life on earth?
Has Four Bonding Electrons
Unique Strong Covalent Bonds
Strong Single, Double and Triple Bonds
Average Bond Energies (KJ mol-1)
C-C
607
Si-Si 230
C-H
416
Si-H 323
C-N
754
Si-N 470
C-O
336
Si-O 368
08/04/2015
O-Si-O = Sand and Rocks
6
Simplest Organic molecule
Carbon has 4 valence electrons
H
H
H C H
H C H
H
H
Ne
Neon
methane
H
C
Stable Octet required
08/04/2015
Covalent Bonding – Atoms Share Electrons
7
C(6) - 1s2, 2s2, 2px1, 2py1, 2pz0
lowest energy
state
2
1
C(6) - 1s , 2s ,
1
2px ,
1
2py ,
1
2pz
Excited state
4 sp3 Hybridization
+
2s
08/04/2015
+
+
2py
2px
2pz
4 X sp3
8
Px
Px
Pz
Pz
Py
08/04/2015
Py
9
1s
2s
2px 2py 2pz
Promotey
sp3 sp3 sp3 sp3
Hybridize
x
109.5o
z
Methane: Carbon
08/04/2015
10
Methane building blocks
08/04/2015
11
Methane is Tetrahedral
0
109.5
H
C H
H
H
Sp3 hybridized carbon
4 equivalent C-H bonds (s-bonds)
All purely single bonds are called s-bonds
08/04/2015
12
120
H
o
H
C
H
C
H
2
2
Flat molecule – “Planar”
1
C(6) - 1s , 2s , 2px ,
1
2py ,
0
2pz
Hybridization
2
1
C(6) - 1s , 2s ,
H
C C
H
p-bond
08/04/2015
H
1
2px ,
1
2py ,
3 sp2
H
1
2pz
p-bond
Three s-bonds
s-bonds – One C-C, two C-H bonds per carbon atom
13
s-bond
s-orbital
p-orbitals
Spherical Symmetry
end to end overlap of orbitals leads to s-bond
p-bond
side ways overlap
When a single bond is
present between atoms, that
bond is always s-bond
DB contains one s-bond and
one p-bond
The p-bond lies perpendicular to the s-bond –
overlapping lobes above and below the plane of sbond
Groups or atoms can be rotated about a single bond, but DB
is rigid – No rotation about a DB is possible without breaking
the08/04/2015
p-bond – This leads to cis-trans Isomerism
14
Linear Molecule
180o
Alkynes
H
C C H
Ethyne
(acetylene)
H3C
C C H
Propyne
Sp3 hybridisation in Saturated Bonds (e.g. alkanes)
Sp2 hybridisation in DB (e.g. alkenes)
Sp hybridisation in TB (e.g. alkynes)
2
2
1
C(6) - 1s , 2s , 2px ,
1
2py ,
0
2pz
Hybridization
2
1
C(6) - 1s , 2s ,
Two s-bonds (C-H) and (C-C)
And Two p-bonds between C-C
08/04/2015
per C atom
1
2px ,
2sp
1
2py ,
1
2pz
2p-bonds
15
ALKANES
08/04/2015
16
Alkanes
CnH2n+2
consist of only carbon and hydrogen bonded by
single covalent bonds single
H
H C H
H
methane
CH3
08/04/2015
H H
H C C H
H H
ethane
CH3CH3
H H H H
H C C C C H
H H H H
H H H H H
H C C C C C H
H H H H H
propane
butane
pentane
CH3CH2CH3
CH3CH2CH2CH3
H H H
H C C C H
H H H
CH3CH2CH2CH2CH3
17
Skeletal structure of only carbon atoms
propane
butane
pentane
C1 – C4 n-alkanes are all gases
Methane main component of natural gas
Propane and butane often stored as compressed gases
08/04/2015
18
Rotation about single covalent bonds
occurs freely. The energy barrier is small.
The position of hydrogen atoms relative to
one is thus constantly changing
H
H
H
C
H
C
H
H
Ethane
08/04/2015
19
Nomenclature
* General Formula
CnH2n+2
Number of
carbon
Name
Structure
One
Two
Three
Four
Five
Sex
Seven
Eight
Nine
Ten
Methane
Ethane
Propane
Butane
Pentane
Hexane
Heptane
Octane
Nonane
CH4
C 2H 6
C 3H 8
C4H10
C5H12
C6H14
C7H16
C8H18
C9H20
C10H22
08/04/2015
Decane
20
Alkyl groups (R): (-H)
# Methane
# Ethane
# Propane
CH4
C2H6
08/04/2015
Methyl
Ethyl
C3H8 (2 R)
CH3-CH2-CH3
- CH2-CH2-CH3
n-Propyl
CH3
C2H5
OR
CH3-CH-CH3
Isopropyl
21
# Butane (C4H10)
Butane
n -butane
CH3-CH2-CH2-CH3
n-butyl
CH2-CH2-CH2-CH3
Isobutyl
CH3
CH2-CH-CH3
08/04/2015
Iso butane
CH3
CH3-CH-CH3
2 butyl
CH3-CH-CH2-CH3
3 butyl
CH3
CH3-C-CH3
22
Degree of carbon
4º
4ry
quat.
1º
1ry
Pry.
08/04/2015
3º
3ry
ter.
2º
2ry
Sec.
23
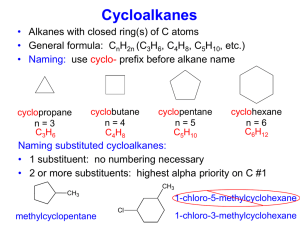
isopropyl
3ry-butyl
2ry -butyl
methyl
isobutyl
08/04/2015
n-propyl
24
International Union of Pure and
Applied Chemistry IUPAC
1-Longest continuous chain
CH3–CH2–CH–CH2–CH3
CH2
CH2
CH3
3-Ethyl hexane
08/04/2015
Not
CH3–CH2–CH–CH2–CH3
CH2
CH2
CH3
3-Propyl pentane
25
2-Lowest number of attachment of substation
CH3–CH2–CH–CH2–CH3
CH2
CH2
Not
CH3
3-ethyl hexane
08/04/2015
CH3– CH2–CH–CH2–CH3
CH2
CH2
CH3
4-ethyl hexane
26
3-Same alkyl substitute (di -, tri-, tetra, penta.. etc.)
CH3
CH3 – CH – CH2 –C – CH3
CH3
CH3
2, 2, 4-Trimethylpentane
Not
2,4,4-Trimethyl pentane
08/04/2015
27
4-Different alkyl substation ( alphabetical)
CH3
CH2
CH3 –CH2 – CH2 – CH – CH – C – CH2 – CH3
CH2 CH3 CH2
CH2
CH3
CH3
3,3-Diethyl-4-methyl-5-n-propyloctane
08/04/2015
28
CH3
CH2
CH3 –CH2 – CH2 – CH – CH – C – CH2 – CH3
CH2 CH3 CH2
CH2
CH3
CH3
08/04/2015
29
Name the following compound
CH3CH3
CH3CH2CH2CH2CH
C C CH3
H H
CH2 H
CH2 C CH3
CH3
longest chain = 9 carbons = nonane
1,2-dimethyl propyl substituent
methyl
need to be in alphabetical order
5-(1,2-dimethylpropyl)-2-methylnonane
3D – models show that because of the
tetrahedral carbon atoms the chains are
zig-zagged and not at all straight
08/04/2015
30
Physical Properties of Alkanes
Non-polar molecules, which are less dense than
water.
Alkanes are immiscible with water making two
layers.
Van-der Waals or dipole-dipole attractive forces,
and not H-bonding (as in polar molecules) are
the main intermolecular forces
Alkanes show regular increases in bpt and mpt
as molecular weight increases down the
homolgous series
08/04/2015
31
Physical Properties of Alkanes
These weak intermolecular forces operate over
small distances, arising because the electron
distribution within molecules at any given
instance is not uniform. Resulting in tiny
electrical attractions between molecules.
These temporary dipoles hold alkanes
as liquids or solids, and must be
overcome in order to vaporize a liquid or
melt a solid (wax)
08/04/2015
32
Isomers – they have the same molecular formula, but a
different structures Structural Isomers – same molecular
formula, but atoms are bonded in different orders
H
.
H3 C
C
CH3
C4H10 – has two isomers, n-butane and
isobutane (2-methylpropane)
CH3
Isobutane
H3 C
CH
CH2
CH3
Isopentane
CH3
Has the same molecular
formula as n-pentane, C5H12
(2-methylbutane)
CH3
H3 C
C
CH3
CH3
Neopentane
08/04/2015
Have different Physical
Properties, Mpt, Bpt, densities,
(2,2-dimethylpropane)
33
Fractional distillation of crude oil
Natural Gas (C1-C4)
Gasoline (C4-C12)
Bpt (40-200 ºC)
Petroleum
Kerosene (C12-C16)
Bpt (200-250 ºC)
Heating oil (C15-C18)
Bpt (250-300 ºC)
Straight-chain alkanes are a pure fuel, because of engine knock.
n-Heptane has an octane rating = 0
Catalytic cracking
2,2,4-trimethylpentane has an octane rating = 100
08/04/2015
34
CYCLOALKANES and
Conformational Analysis
08/04/2015
35
Cycloalkanes
H2C CH2
C
H2
H
CnH2n
H
C C H
H
C
H
H
Cyclopropane
H2C CH2
H2C CH2
H2C CH2
H2C
CH2
C
H2
08/04/2015
Cyclobutane
Cyclopentane
36
Angle Strain in Cyclopropane and Cyclobutane
– weaker “Bent” C-C bonds C-C Bond angles 60
and 88o respectively
Eclipsed hydrogens – Torsional Angle
Reduced in Cyclobutane by folding or bending
Pentane has C-C bond angles of
108o C-C bonds slightly bent out of planarity in order
to reduce torsional strain
The most stable cycloalkane with
109.5o C-C bond angles
Cycloalkanes
have higher bpt/mpt than straight chain
08/04/2015
alkanes with the same number of carbon atoms
37
Sir D.H.R. Barton, Nobel Prize 1969
08/04/2015
38
How to draw Cyclohexane ?
H
H
H
put in axial H’s
H
put in equitorial H’s
H
H
H
H
H
H
H
H
08/04/2015
H
H
H
H
H H
H
H
H
H
H H
H
H
39
Reactions of Alkanes
Combustion
CH4
+ 2O
2
CO2
+ 2 H O + energy
2
Dehydrogenation
RCH2
High Temp.
catalyst
CH2R
RHC
CHR
alkene
light or heat
08/04/2015
+ Br2
+
H
H
Br
+ HBr
40
08/04/2015
41
When bonds break ions are created – driven by
the energy of solvation
Each atom gets one electron each – results in
the formation of radicals
Radical – neutral species with one unpaired
electron
Using Curly Arrows
08/04/2015
42
Sir Robert Robinson, Nobel Prize 1947
Introduced curly arrows in 1922, numerous
brilliant syntheses of complex natural products
08/04/2015
43
Halogenation
Substitution Reaction – a reaction in which part
of a small reacting molecule replaces an atom or a
08/04/2015
group of atoms on the organic molecule
44
Mechanisms are widely used by organic chemists to explain reaction
pathways to observed reaction products
Initiation
Two highly reactive Chlorine radicals formed
Hydrogen abstraction to form a methyl radical
08/04/2015
45
Propagation
Chlorine atom is abstracted to form a chlorine radical
Propagation are the product forming steps
Chain Reaction – thousands of radical forming cycles
08/04/2015
46
Termination
Radicals Couple
Product forming Chains
are broken
As the reaction progresses chloromethane
accumulates and its hydrogen atoms can
be abstracted.
08/04/2015
47
Fluorine is the most reactive halogen – mixtures of
fluorine and methane can be explosive. Fluorine radical is
very reactive. The reaction is controlled with the addition
of an inert gas to dilute the reaction.
Chlorine is next most reactive, followed by bromine.
Cl2 and Br2 require heat or light. Iodine does not react
with methane easily. Iodine radical is disperse and
large - unreactive
08/04/2015
48
Alkyl Halides or Haloalkanes
08/04/2015
49
Naming them
Tend to be Heavier than water
More Toxic than Alkanes
Cl
Cl
C
CH3 CH
Cl
CH
Cl
Cl
Tetrachloromethane
or carbon tetrachloride
CH3
CH3 CH
CH3
CH2 CH2
Br
2-Chloro-3-methylbutane
Cl
3-Bromo-1-chlorobutane
CH2CH3
Br
F
Cl
1-Bromobutane
1-Ethyl-2-fluorocyclohexane
Cl
Cl
C
F
F
Cl
Trichlorofluoromethane
(Freon-11)
Cl
C
Cl
F
F
2-Chloropropane or
Isopropyl chloride
F
F
C
C
F
H
H
Dichlorodifluoromethane
1,1,1, 2-Tetrafluoroethane
(Freon-12)
Chlorofluorocarbons
(CFCs)
08/04/2015
Refrigerant Gases, Ozone Depletion, More H’s more degradable
50
X dd+
C
X = F, Cl, Br
X is readily displaced by nucleophiles
Nu Electro negativity is defined as the ability of atoms to attract shared
electrons in a covalent bond ------------ leads to nucleophilic
substitution in alkyl halides
Cl
Cl C Cl
Cl
08/04/2015
Symmetrical molecules have no dipole
moment or have equal distribution of
electrons within covalent bonds
Therefore, they are unreactive!
51
ALKENES
08/04/2015
52
Unsaturated Compounds – contain DB and or TB
ALKENES
H2C
CH2
Ethene
1-Butene
End in ene
H3C
CH
CH2
CnH2n
Propene
1-Pentene
1-Hexene
1,3-Butadiene
3-methyl-1,4-pentadiene
08/04/2015
53
H
120o
H
C
C
H
Flat molecule – “Planar”
H
2
2
C(6) - 1s , 2s ,
1
2px ,
1
2py ,
0
2pz
Hybridization
2
1
1
C(6) - 1s , 2s , 2px ,
H
H
C C
H
1
2py ,
3 sp2
H
1
2pz
p-bond
Three s-bonds
p-bond
08/04/2015
s-bonds – One C-C, two C-H bonds per carbon atom
54
s-bond
s-orbital
p-orbitals
Spherical Symmetry
end to end overlap of orbitals leads to s-bond
p-bond
side ways overlap
When a single bond is
present between atoms, that
bond is always s-bond
DB contains one s-bond and
one p-bond
The p-bond lies perpendicular to the s-bond –
overlapping lobes above and below the plane of sbond
Groups or atoms can be rotated about a single bond, but DB
is rigid – No rotation about a DB is possible without breaking
the08/04/2015
p-bond –
55
This leads to cis-trans Isomerism
If each of the two carbons has two different groups attached to it
Geometric isomers have
different chemical &
physical properties
H3C
H
CH3
H
H3C
H
cis-(Z)-2-butene
H
R
R
R
H
H
H
H
R
cis-
trans-
CH3
trans-(E)-2-butene
Z-E system, we take the group with higher priority (here
higher molecular weight), and compare it with the group
with higher priority on the other carbon
08/04/2015
56
Cl
Br
F
H
Cl > F
H3C
H
Br > H
(Z)-2-Bromo-1-chloro-1-fluoroethene
F
Cl
H
F > CH3
CH3
CH3 > H
(Z)-2-fluorobutene
F
Br
Cl > F
Br > H
(E)-2-Bromo-1-chloro-1-fluoroethene
08/04/2015
57
p-bond lobes represent areas of high electron density
Therefore, the p-bond is susceptible to attack by
electron deficient molecules, called electrophiles, E+
ADDITION REACTIONS
E+
C
H
H
C
X
OSO 3H
+
C
H
H
A
C
C
C
C
A
B
X
OSO 3H
C
C
Alkyl Halides
Alkyl hydrogen Sulfate
C
H
OH
H+
08/04/2015
X
H
X
C
X
C
C
OH
C
Alcohols
X
Dihaloalkanes
58
B
Mechanism
Slow
C
C
C
C
+ X
H
H
X
fast
C
H
C
X
C
C
H
X
X- is the nucleophile, and the carbocation is the electrophile
The electrophile is a Lewis acid, its accepted a pair of electrons,
the simplest Lewis acid is H+
The nucleophile is a Lewis base, its donated a pair of electrons
08/04/2015
59
C
Slow
C
C
O
C
O
H
H
OSO 2OH
Markovnikov’s Rule
H2 C
H
Br
CH3
H2 C
H
Unsymmetrical Alkene
08/04/2015
O
H
O
Fast
H
C
S
C
C
H
OSO 3H
H atom adds to the carbon atom
which already has the most H
atoms
H
C
CH3
Br Markovnikov addition
Product
60
H
CH3
+C
H
H
+C
H
CH3
CH3
H
+C
H
CH3
+C
CH3
CH3
INCREASING STABILITY OF CARBOCATIONS
This determines the selectivity
of addition of HX onto an
unsymmetrical alkene
08/04/2015
61
H
CH3
C
C
H
H
2-Bromopropane
is the main product
BrH2C
C
H
H2
C CH3
C
C
H
Br
2o Carbocation prefered
C
H
H
CH3
H
CH3
1-Bromopropane – little formed
08/04/2015
Slow
H
Br
H
CH3 CHBr CH3
H
H
Br
Slow
CH3
H
C
H
C
H
H
1o Carbocation
3o > 2o > 1o
Carbocation stability
The Slow Step is the Rate Determining Step
62
Bromination of DB – This is an Ionic Mechanism
C C
C C
Br
Br d+
Br d-
+
Br
Test for DB or TB
Decloroization of Br2/CCl4
1. Bromine molecule becomes polarised
2. Bromine bond breaks heterolytically
3. Formation of Bromonium cation and Bromide anion
Br
C C
Br
Br
C C
Contrast with reaction
between Bromine and
Alkanes
Br
colourless
4. Back-side nucleophilic attack – opening of three membered ring
5.
Stereospecific Product
08/04/2015
63
Hydrogenation – “Reduction”
C
H
C
H
C
C
Example
2X
H
Pt or Pd - catalyst
solvent, pressure
C H
Pt - catalyst
solvent, pressure
H
H C H
H C H
Pt
ethanol, 1 atm
cyclohexene
08/04/2015
C H
cyclohexane
64
Oxidation of Alkenes
H2C CH2
KMnO4, -OH, H2O
H2C CH2
+ MnO2
OH OH
1,2-Ethanediol
LEO Says GER
Oxidation =
Loss of electrons
Loss of Hydrogen
Gain of Oxygen
08/04/2015
Reduction =
Gain of electrons
Gain of Hydrogen
Loss of Oxygen
An oxidizing agent gets reduced
A reducing agent gets oxidized
65
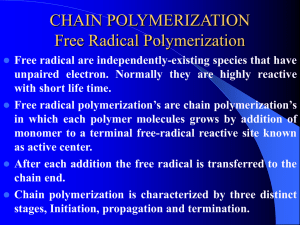
H
H
C C
Polymers are large
molecules containing
many identical repeating
units (100-1000000)
H
H H
ethylene
H
C C
H H
H Cl
n
n
C C
Addition polymer is a
polymer in which the
monomer simply add
together with no other
products formed besides
polymer
08/04/2015
H
F
C C
F n
Teflon
H
C C
F
F
F
F
Tetrafluoroethene
H
C C
H H n
Poly(vinylchloride), PVC
vinyl chloride
F
n
Polypropylene
H
F
C C
H
Cl
H
H CH3
n
C C
H
n
Polyethylene
CH3
H
C C
H
propylene
Polymerisation reaction is a repetition
reaction which combines many small
molecules of monomer (alkene) to
form a polymer
H H
n
n
H H
C C
H
n
66
Styrene
Polystyrene
ALKYNES
08/04/2015
67
Linear Molecule
180o
Alkynes
H
C C H
Ethyne
(acetylene)
H3C
C C H
Propyne
Sp3 hybridisation in Saturated Bonds (e.g. alkanes)
Sp2 hybridisation in DB (e.g. alkenes)
Sp hybridisation in TB (e.g. alkynes)
2
2
C(6) - 1s , 2s ,
1
2px ,
1
2py ,
0
2pz
Hybridization
2
1
1
C(6) - 1s , 2s , 2px ,
Two s-bonds (C-H) and (C-C)
And Two p-bonds between C-C
08/04/2015
per C atom
2sp
1
2py ,
1
2pz
2p-bonds
68
Therefore, a Triple bond consists of one s-bond and two pbonds
The two p-bonds are perpendicular to each other and
form a cylinder of negative charge about the axis of the
bond ---------- No bond rotation about TB
sp-orbitals contain 50% s- and 50% p-character
Far less disperse than sp2, which is less disperse than sp3
The Carbon-Carbon bond is 1.2Ao shorter than C=C, which is
1.3Ao. C-H bond is also shorter than ethene, which is shorter
than ethane, because in ethyne it is overlap between an sp
orbital and a s-orbital of H to give the s-bond.
The08/04/2015
bonding electrons reside closer to the C-nucleus, and
so
69
are held more tightly.
Alkynes are more reactive in halogenation reactions than
alkenes (no longer in this course) and --------
Combustion
Alkynes are high energy compounds
H C C H
+
2.5 O2
2 CO2 + H2O
Welding gas
08/04/2015
70
Benzenes & AROMATICS
08/04/2015
71
An Aromatic Hydrocarbon is a cyclic compound that does not
readily undergo addition reactions
Reactivity is different to other unsaturated compoundsSubstitution rather than Addition is favoured.
H
Benzene
C6H6
H
H
C
C
C
C
C
C
H
H
H
High Carbon content –
burn with a smoky flame
Resonance Structure
- Rearrange the bonding electrons
Delocalisation, Resonance
-Stabilise molecules, so make
them less reactive
Delocalised
or Conjugated System – p-bonding
08/04/2015
electrons can move within the molecule
72
H
H
H
C
C
C
C
C
C
H
H
Kekul said that he dreamt
the structure of benzene –
so called Kekul structure
of benzene
H
In aromatic compounds the C atoms are sp2 hybrids, so that each
C atom has one remaining p-electron involved in p-bonding
Three sp2 hybrid orbitals arrange themselves as far apart as possible which is at 120° to each other in a plane. The remaining p orbital is at
right angles to them.
Each carbon atom uses the sp2 hybrids to form s-bonds with two other
carbons and one hydrogen atom.
This extensive sideways overlap produces a system of p-bonds which are
spread out over the whole carbon ring. Because the electrons are no longer
08/04/2015
73
held
between just two carbon atoms, but are spread over the whole ring, the
electrons are said to be delocalised.
=
Flat (Planar) Molecule
Regular Hexagon
p-Electron Density Rings above and below
the plane of the ring – Susceptible to
electrophilic attack
Benzene is a colourless odourless liquid
that
is a suspected
carcinogen
Benzene
and its derivatives
are said
to be aromatic - a term coined
because of the strong fragrance of
some of the derivatives of benzene
Non-aromatic compounds are
said to be aliphatic
08/04/2015
Michael Faraday first
isolated benzene in 1825
74
=
Flat (Planar) Molecule
Regular Hexagon
Delocalised or Conjugated System
– p-bonding electrons can move within the
molecule
08/04/2015
75
1. Must be cyclic
Rules for Aromaticity
2. Must be planar
3. Each atom of the ring must have a p orbital and these p orbitals must
be perpendicular to the plane of the ring
4. Must contain 4n+2
Rule
p-electrons (where n = 0, 1, 2, ...) –Hückel
n = 1 , 6pelectrons
Naphthalene
08/04/2015
10 π
Anthracene
Phenanthrene
14 π
76
Vinyl group
Br
CH3
1
2
O
3
4
O
08/04/2015
+
N
O
H
N
m
p
H
OH
HO
O
77
Naming Aromatic Hydrocarbons
F
CH2CH3
Fluorobenzene
NH2
CH3
Ethylbenzene
Toluene
Aniline
O
OH
Cl
C
OH
-ortho
Cl
Cl
-meta
Phenol
Cl
Benzoic Acid 1,2-Dichlorobenzene 1,3-Dichlorobenzene
CH3
-para
Cl
O 2N
CH3
NO2
CH3
Br
08/04/2015
NO2
Cl
1,4-Dichlorobenzene
o-Xylene
m-Bromostyrene
2,4,6-Trinitrotoluene (TNT)
78
Electrophilic Aromatic Substitution
H
E
H
H
E
H
X
H
+
H
H
H
H
H
X
H
H
Electrophilic attack – Slow Rate Determining Step
E
H
E
H
E
H
E
sp3 Transition State or Wheland Intermediate
E
08/04/2015
H
Delocalised Cyclohexadienyl cation
79
Fast Step is the loss of a proton
+ E
---rapid re-aromatization
- H+
E
H
E.g. Nitration of benzene
HNO3(c), H2SO4(c)
NO2
Sir Christopher Ingold's ideas (1930s), terminology and
nomenclature for reaction mechanisms (e.g. electrophilic,
nucleophilic, inductive, mesomeric, SN1, SN2 etc) were
generally accepted and employed everywhere.
08/04/2015
80
The Nitration of Benzene
_
O
+
N
electrophilic attack
O
electrophile
O2 N
+
- H+
fast
08/04/2015
O
O
+N
+
slow
H
_
O +
N
O
O
+N
=
+
O
NO2
=
81
Generating NO2+
Sulfuric acid is a stronger acid than nitric acid
O
_
H O S O H
O
_
O S O + 2 H+
O
O
H
HO NO2
H+
H
O+ NO2
NO2+
NO2
+
H2 O
NO2
H
NO2
- [H+]
Nitrobenzene
08/04/2015
82
HALOGENATION
Cl2, AlCl3
Cl
Br2, FeBr3
Br
Professor Charles Friedel
and Professor James Crafts
The Halogen is polarised
Br
Br
08/04/2015
Br FeBr3
H
+
FeBr4
83
Conclusions
Aromatic Compounds are resonance stabilized
This gives them added stability
They undergo Electrophilic Substitution Reactions
Upon substitution, the fast step is the loss of a proton to regenerate
aromaticity
H
Br
+
Br
H
H
+
Br
+
FeBr4
Br
double-headed arrows
+
HBr
FeBr3
08/04/2015
Regenerate the catalyst – so only a small amount is required
84
Diazonium Coupling Reactions
mauve
Azo Dyes
William Perkin
Write the mechanism for the formation of mauve from the
diazonium salt of aniline
08/04/2015
85
ALCOHOLS, PHENOL and
ETHERS
08/04/2015
86
Alcohols and Ethers
Alcohols and Ethers can be regarded as derivatives of water
in which one or two of the H atoms has been replaced by an
alkyl group
Methanol, CH3OH
Water, H2O
H
O
0.96 Ao
o
0.96 A
H C
H
O
H
H H
104.5o
o
1.43 A
O
C H
H H H H
o
109.5
08/04/2015
111.7o
1.43 A
Saturated molecules
are sp3 hybridized
108.5o
Methoxymethane, CH3OCH3
H C
o
1.10 Ao
- I (net dipole)
dO d+
H3C
H
Electronegativity of oxygen causes an
unsymmetrical distribution of charge
87
Alcohols are found to have much higher bpt than those of alkanes or
haloalkanes of comparable size, e.g. Methanol (65 oC),
Chloromethane and Methane are gases ; Ethanol (78.5 oC),
Chloroethane (12 oC) and Ethane is a gas
Methanol and Ethanol are classed as Polar Molecules (Hydrophilic)
– They are Infinitely Soluble in Water
Why? Answer – Hydrogen
R
H
Bonding
H
R
O dH
d+
H
O
H
O
R
H
O dH
d+
H
O
O
H
H-bonds weaker than covalent bonds, although these bonds can be
continually broken and reformed – a highly ordered structure
results – H-Bonding to water can also occur
08/04/2015
Water (mw = 18) is a liquid, bpt 100oC – otherwise a gas
88
Ethanol
H H
1-Pentanol
H C C O
H C C C C C O
H H
Hydrophilic end
H H H H H
H
H H H H H
As R-group increases in size,
so does the solubility in nonpolar solvents
H
Hydrophobic end
As the number –OHs increases so does solubility in water
Bpt increase with chain length and number of –OHs
Methanol, CH3OH
In the Liver
- Solvent in varnishes, paint
- Racing Car Fuel (easy to put
out flames)
- Highly Toxic – “Blindness” Formaldehyde
In
Ethanol, CH3OH
-Drinking Alcohol
H3C
O
OH
H
C
H
Alcohol Dehydrogenase
the Liver
CH3CH2OH
O
H3C
Alcohol Dehydrogenase
C
O
[O]
H
Acetaldehyde
- 50% Ethanol is
08/04/2015
flammable
OH
O
[O]
H
C
H3C
C
OH
Acetic Acid
89
Odour on your breath
Symptoms - Hang-over
Ethanol content; Beer, 3-9% ; Wine, 11-13% ; Whisky, 40-45% ; Vanilla Extracts,
35% ; Night Nurse, 25% ; Listerine, 25%
Preparation of Ethanol
- Fermentation of Sugar – Break down of sugar to CO2
and Ethanol by Yeast Enzymes
- Industrial Process – Hydration of Ethene
H
H
H
H2O
CH3CH2OH
H H3PO4 , 300C
Naming Alcohols
hydroxy or alcohol group
CH3 OH
CH3
CH2
OH
CH3
Methyl alcohol
Ethyl alcohol (ethanol)
(methanol)
CH3
08/04/2015
CH OH
CH3
Isopropyl alcohol
CH3
CH2
CH2
CH2 OH
Propyl alcohol (propanol)
CH CH2 CH3
CH2 OH
2-Ethyl-1-butanol
90
Naming Alcohols
Polyhydroxy alcohols are alcohols that possess more than one
hydroxyl group
CH2
CH CH2
CH2
HO
CH2
CH3
CH CH2
HO
OH
OH
HO
HO
OH
1,2,3-Propanetriol (glycerol)
1,2-Propanediol (propylene glycol)
1,2-Ethanediol (ethylene glycol)
Harmless
Extremely Toxic
Calcium Oxalate
crystallises in the kidney
leading to renal problems
08/04/2015
CH3
O
CH2
HO
CH2
OH
HO C
Liver Enzymes
HO
OH
H 3C C
Liver Enzymes
C OH
Oxalic acid
O
CH CH2
O
O
C OH
91
Pyruvic acid
CH3
H
CH3
H3C C OH
H3C C OH
H
H
Primary (1o) Alcohol
H3C C OH
CH3
o
Secondary (2 ) Alcohol
Tertiary (3o) Alcohol
Alcohols are very weak Acids
H
R O H
d+ d- d+
H
+
R O
Alkoxide
O H
Alcohol
Relative Acidity ; H2O > ROH > R
2 CH3CH2OH + 2 Na
08/04/2015
2 CH3CH2
Vigorous Reaction
H O H
C C H > RH
O
Na
+ H2
92
STEREOCHEMISTRY
08/04/2015
93
Isomers are different compounds that have the same molecular formula
Structural isomers are isomers that differ because their atoms are
connected in a different order
CH3OCH3 ---- dimethyl ether and
CH3CH2OH ---- ethanol
Stereoisomers differ only in the arrangement of
their atoms in space
Geometric Isomers
Cl
H
Cl
H
C2H2Cl2
Cl
08/04/2015
H
cis-1,2-Dichloroethene
H
Cl
trans-1,2-Dichloroethene
94
Enantiomers are stereoisomers whose molecules are
nonsuperimposable mirror images of one another
Objects that are superimposable on their
mirror images are said to be achiral
Involves a tetrahedral sp3 atom
H
CH3
C
CH2
CH3
OH
Chiral Centre
2-Butanol
CH3
H
C OH
Interchanging any two groups at a chiral
CH2
CH2
different groups converts one enantiomer
CH3
CH3
into another
CH3
HO
H
C
08/04/2015
centre (stereocentre) that bears four
95
One structure can be superimposed on another
CH3
H C OH
CH3
CH3
HO C H
CH3
If any of the groups
attached to the
tetrahedral atom are
the same, the centre
is achiral.
2-Propanol
The ultimate way to test for
molecular chirality is to construct
models of the molecule and its
mirror image and then determine
whether they are superimposable
Screwdriver is achiral
Socks are achiral
Golf club is chiral
Gloves are chiral
A molecule will not be chiral if it possess a
centre or plane of Symmetry
08/04/2015
96
Properties of Enantiomers
Enantiomers have identical melting points and boiling points
Enantiomers have identical solubilities in solvents
Enantiomers have identical spectra and refractive index
Enantiomers interact, and react with achiral molecules in the
same manner
Enantiomers interact and react with other
chiral molecules at different rates
Enantiomers rotate plane-polarised light by
equal amounts but in opposite directions
Chiral molecules are
optically active
Plane-polarised light
Oscillation of electrical field of ordinary light
occurs in all possible directions
Polarimeter is a devise used to measure the effect of plane08/04/2015
polarised
light on an optically active compound
97
No Correlation between the direction of rotation of plane
polarised light and the absolute configuration of a molecule
Clockwise Rotation (+) – dextrorotatory
Anti-Clockwise Rotation (-) – levorotatory
Same Configuration
CH3
H2C
C H
HO
CH2CH3
(R)-(+)-2-Methyl-1-butanol
CH3
H2C
C H
Cl
CH2CH3
(R)-(-)-1-Chloro-2-methylbutanol
An equimolar mixture of two enantiomers is called a Racemic Mixture
It is Optically Inactive
08/04/2015
98
O
O
H
S-(+)-Carvone
Principle component of
Caraway seed oil and
responsible for the
characteristic odour
H
R-(-)-Carvone
Principle component
of Spearmint oil and
responsible for the
characteristic odour
Receptor Sites in the Nose are Chiral
08/04/2015
99
Nobel Prize 2001
Professor William Knowles
Professor Ryoji Noyori
Professor K. Barry Sharpless
For synthesis of optically active compounds – asymmetric synthesis
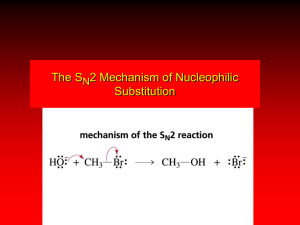
SN2
H3C
HO
H C Br
C6H13
R-(-)-2-Bromooctane
08/04/2015
CH3
CH3
HO C Br
H
C6H13
HO
H
C6H13
S-(+)-2-Octanol
Transition State
Inversion of Stereochemistry
100
SN2 – Substitution, Nucleophilic, Bimolecular
Rate = k2 [R-Br] [Nuc-]
Backside Nucleophilic Attack – Inversion in Configuration
H3C
HO
H C Br
C6H13
R-(-)-2-Bromooctane
CH3
CH3
HO C Br
H
C6H13
Transition State
HO
H
C6H13
S-(+)-2-Octanol
Optically Active
Enantiomericaly Pure
Inversion of Stereochemistry
Concerted Mechanism
08/04/2015
101
SN1 – Substitution, Nucleophilic, Unimolecular
(CH3)3COH + 2 H3O+ + Cl -
(CH3)3CCl + 2 H2O
Slow Step (RDS)
CH3
CH2
H3C C Cl
+
H3C
CH3
Aided by polar Solvent
Cl
CH3
Stable 3o Carbocation
ions are stabilized via solvation
CH2
Professor George Olah
Nobel Prize 1994
H3C C O H
H3C
Carbocation is sp2-planar
R C
>
> R C
R
08/04/2015
H
H
R
CH3
O H
H
Front or Backside Attack
more stable
R
CH3
Fast Step
R C
H
R groups are electron releasing
- delocalise the positive charge
CH3 H
- H+
CH3
H3C C O H
CH3
102
tert-Butyl alcohol
H3CH2CH2C
- HBr
H3C C Br
H3CH2C
S-3-Bromo-3-methylhexane
CH2CH3
HO C CH3
CH2CH2CH3
H3CH2CH2C
+
H3C C OH
H3CH2C
1:1 Mixture of R- and S-3-Methyl-3-hexanol
The Carbocation intermediate is
attacked by water from either
side by the same rate
08/04/2015
103
Phenols are stronger acids than alcohols
OH
OH
pKa = 18
H O
CH3
O
cyclohexene
OH
pKa = 10
O
O
Resonance Stabilised Phenoxide anion
phenol
Tetrahydrocannabinol
H3C
O
CH3
104
08/04/2015
cyclic ether
ETHERS, RO-OR
CH3CH2 O CH2CH3
CH3CH2 O
Diethyl Ether
H3CO
O
Methoxycyclohexane OCH3
H3C O
Methoxy group
Ethoxy group
Non-Flammable Anaesthetics
1-Propoxypropane
Methoxybenzene
“anisole”
Cl F
F
Bpt are similar to alkanes – No H-bonding to one another H C C O C H
But are soluble in water- H-bonding to water - Polar
F F
F
Flammable – Ether can cause flash fires
Enflurane
Low Reactivity – Make Good Reaction Solvents
F H
F
F
O
Furan
08/04/2015
O
Tetrahydrofuran (THF)
Cyclic Ethers
O
Pyran
C C O C H
F Cl
F
Isoflurane
105
ALDEHYDES AND KETONES
08/04/2015
106
Aldehydes and Ketones
H
R
R
C O
Aldehyde
C O
R
Ketone
p-bond - two overlapping 2p orbitals
H
H
C
O
lone Pairs
O 1s2, 2s2 2p2 2p1 2p1
s-bond
3 sp2 orbitals
s-bond- overlapping 1s
H-orbital
and sp2 C-orbital
08/04/2015
107
Useful in Synthesis
H d+ d118o C O
H3C 121o
H
118o
H
C C
H 121o H
Resonance Structures
H
H
Most Reactive Group –
C O
C O
p-electrons + polarisation
H3C
H3C
Names
al – aldehydes, one - ketones
H
H
C O
C O
H
08/04/2015
Methanal
(formaldehyde)
H
H3C
H
C O
CH3CH2
Propanal
Ethanal
(acetaldehyde)
C O
CH3CH2CH2CH2
Pentanal
108
H
O
H
H
Benzaldehyde
O
H
trans-Cinnamaldehyde
S
O
H
Acrolein (2-propenal)
- lachrymator and pleasant "odour"
from barbacuing meat
Formalin, 35-40% formadehyde in water
Preservative that reacts with
proteins causing them to resist decay
08/04/2015
Coelacanth,
“prehistoric fish”
H
Thiopropionaldehyde
(propanethiol)
- lachrymator from chopped onion
109
O
CH3
H
O O
O
H3C
Butadione
OCH3
(butter flavour)
OH
Carvone
Vanillin
(spearmint flavour)
O
O
H3C
Propanone
(ACETONE)
H3C
CH3
Butanone
O
Acetophenone
H3C
C
H2
CH3
O
CH
CH2 CH3
CH3
3-Methyl-2-pentanone
O
CH3
08/04/2015
CH3
Benzophenone
110
Carbonyls readily undergo Nucleophilic Attack
dO
C d+
O
O H
C
C
H N R
H N R
H
RNH2
ANHYDROUS
Conditions are required for
imine formation
O H
C
C
Imine
N
H N R
R
- H2O
Reaction
between an amine and a carbonyl compound
08/04/2015
111
Condensation Reaction – Elimination of water
H
H
H3C
C O
H3C
N N
+
H
H3C
C N N
- H2 O
H
H
H3C
hydrazine
acetone
H
hydrazone of acetone
NO2
- H2O
O2 N
H3C
C O
+
H3C
acetone
H
N N
O2 N
H
H
H3C
NO2
C N N
H3C
H
hydrazone of acetone
2,4-diphenylhydrazine
DNP test for aldehydes & ketones gives crystalline hydrazones
08/04/2015
112
Emil Fischer, Nobel Prize 1902
H
dd+
R C X
Mg
Ether
H
H
d- d+
R C MgX
X = I or Br
RCH2
H
Grignard Reagent
MgX
Professor Victor Grignard (1912 Nobel Prize)
Developed this chemistry with Professor P. A. Barbier
H dd+
R C X
H
d- d+
R C Li
Li
Ether
H
X = I or Br
RCH2
H
Organolithium Reagent
ADDITION
dR
08/04/2015
C O dd+
Li
d+
Li
Protonation
R C O
R C O H
Li
H
H2O
Alcohol
113
Organometallics add to carbonyls to give alcohols
Ether
H
C O MgBr
MgBr
H
H
C O
H3O+
H
H
C O H
C O
+
MgBr
C OH
H
Benzylalcohol
Ether
2. H3O+
Triphenylmethanol
Benzyl Group
Phenyl, Ph Group
08/04/2015
Ph
114
Nucleophilic Addition Reactions
H
O
H C H
Ph
Primary alcohols
O
R C H
Ph
Secondary alcohols
your adding Ph
H
O
C
H
Formaldehyde
+
Ph MgI
08/04/2015
H
H
O
R C R
Ph
tertiary alcohols
_
O
C
R
H
Aldehydes
+
Ph MgI
O
C
R
R
Ketone
+
Ph MgI
115
CH3 CH2
CH 3CH 2
..
O
..
CH2 CH3
Mg
Br
..
O
..
Ethers (Lewis base) stabilize the
Grignard Reagent making it
more reactive
CH2 CH3
Organometallic Reactions must always be done under anhydrous conditions
-d +d
Mg
Br
H
H
Grignards are powerful bases and
will deprotonate water
O
08/04/2015
H
+
_
OH
116
CARBOXYLIC ACIDS and ESTERS
08/04/2015
117
Carboxylic Acids
O
C
pKa = 4 - 5 ,
O
water = 16
C
O H
+
H2O
O
+
H3O
We can distinguish a water-insoluble carboxylic acid and phenol from
an alcohol
O
C
O
O H
Benzoic acid
08/04/2015
+
H2O
NaOH
C
O
Na
Sodium Benzoate
118
Carboxylic Acids
Highly Polar
Low molecular weight acids show Appreciable Solubility in Water
High Bpt – Extensive H-bonds to themselves and water
NAMES
O
O
H
CH3
OH
Methanoic acid
O
OH
CH3CH2
Ethanoic acid
OH
Propanoic acid
O
O
HO
Red ants
rhubarb
O
HO
O
( )n
C
C
OH
OH
O
Br
4-Bromo-2-ethylpentanoic acid
Ethanedioic acid (oxalic acid)
OH
n = 1 = malonic acid
n = 2 = succinic acid
08/04/2015
n
= 3 = glutaric acid
HO2C
CO2H
Terephthalic acid
CO2H
CO2H
Phthalic acid
119
Esterification – condensation reaction, where H O is lost
2
O
O
+ CH3CH2
OH
CH3
Acetic acid
(ethanoic acid)
OH
CH3
Ethyl acetate
HCl or H 2SO4
H+(catalyst)
O
O
+
OH
Ph
H3C OH
Benzoic acid
Ph
H+(catalyst)
O
Ethyl propanoate
O CH3
Methyl benzoate
O
O
O
O
O
Methyl formate
vinyl acetate
H
Alcohol part appears first in the name
08/04/2015
O CH2 CH3
120
Ester molecules cannot H-bond to each
other, because they do not have an –OH
Consequently, B.pt is much lower than that
of alcohols and acids of comparable mass
H-bonding to water is possible
-low mw esters are soluble in water
Solubility rapidly decreases with carbon
chain length.
H O
H
O
R
08/04/2015
O
R
H O
H
121
Highest Boiling points and exceedingly water soluble
O
R C
O R
O........ H O
R C
C R
O H ........ O
Two hydrogen bonds
cannot H-bond to another ester molecule
Boiling points
Hexane = 69 ºC
Diethyl ether = 56 ºC
Ethanol = 78 ºC
Ethanoic acid = 118 ºC
Ethyl acetate = 77 ºC
08/04/2015
122
Redox Reactions
Addition of Oxygen or Removal of Hydrogen is OXIDATION
Removal of Oxygen or Addition of Hydrogen is REDUCTION
CH4
+O
CH3OH
- 2H
H
C O
H
R
Reduction
R
Oxidation
Reduction
08/04/2015
Ketones
C O
- 2H
HO
O
C
O
H
H
Primary Alcohols
H
R C O
C O
R
H
H
R C O
C O
H
Aldehydes
+O
Oxidation
H
R
Secondary Alcohols
123
Examples of Reduction Reactions
CH3 O
H3C
CH3 O
H2 , Pd-C
H
H
H3C
3-Methylbutanal
O
H
3-Methylbutanol
H
O
H
H
H2 , Pt
Cyclohexanone
Cyclohexanol
Examples of Oxidation Reactions
OH
H
Overoxidation
K2Cr2O7, H2SO4, H2O O
08/04/2015
OH
O
124
Organic bases are amines
Amines are derivatives of ammonia
H N H
R N H
H
Ammonia
H
Primary (1o) Amine
R N H
R N R
R
R
Tertiary (3o) Amine
Secondary (2o) Amine
08/04/2015
N 1s2, 2s2 2p1 2p1 2p1----------- lone pair
occupies an sp3 orbital
125
AMINES, AMIDES and ANILINE
08/04/2015
126
Ammonia
..
N
H
107O
..
3oAmine
H
N
H
R
R
R
107O
Unshared lone pair of electrons in the fourth sp3 hybrid occupies
slightly more space than the electrons in the s-bonds
08/04/2015
127
Naming amines
where Et = CH2CH3
H2NEt
ethylamine
primary
HNEt2
NEt3
diethylamine triethylamine
secondary
tertiary
where Me = CH3
H2NMe
methylamine
primary
H N
08/04/2015
HNMe2
dimethylamine
secondary
NMe3
trimethylamine
tertiary
methylpropyl amine
128
Some Common Amines
1,4-butanediamine
NH2
H2N
Putrescine
(found in decaying meat)
NH2
Both upper amines are 1o
Amphetamine
(dangerous stimulant)
N
N
Isopropylamine
H
Triethylamine
Piperidine
This amine is are
08/04/2015
2o
This amine is 3o
NH2
This amine is 1o
129
Amines are bases because of the lone pair on the
nitrogen atom - red litmus paper to blue
H
H
Cl
NH2
Base
+
Acid
N H Cl
H
Ammonium Salt
=
O
H O
O
O H
O
oxalic acid
08/04/2015
+ 2 N(CH2CH3)3
triethylamine
O
O
+
2 HN(CH2CH3)3
O
triethylaminium oxalate
130
Aniline is useful in the synthesis of many other aromatic compounds
HNO3, H2SO4
NO2
NH2
NO2
Sn, HCl
phenylamine
08/04/2015
= aniline
131
Aniline can be converted into useful diazonium salt
NH2
NaNO2, HCl
N N + Cl-
0C
benzenediazonium chloride
N N + Cl-
08/04/2015
Nuc-
Nuc
N N
132
N N + Cl+ KCN
CN
CuCN
-
N N
Benzene nitrile
N N + Cl-
NaI
N N + Cl-
08/04/2015
N N
HBr, CuBr
-
I
iodobenzene
Br
N N
bromobenzene
133
Amides
R'
R'
N
C
O
------------- Not acids or bases
R
N
C
R
O
Features of a Peptide Bond;
1. Usually inert
2. Planar to allow delocalisation
3. Restricted Rotation about the amide bond
4. Rotation of Groups (R and R’) attached to the amide
bond is relatively free
08/04/2015
134
R
H2N C COOH
H
AMINO ACIDS
O
C
H
NH2
formamide
H3C
O
C
O
NH2
NH2
acetamide
benzamide
O
C
H2N
NH2
urea
All are high melting point solids, only
benzamide not soluble in water
08/04/2015
135