3. Biological membranes and cell compartments
advertisement

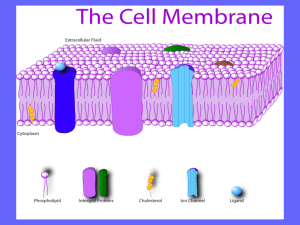
3. Biological membranes and cell compartments Structure of lipid bilayers Structure of membrane proteins Membrane dynamics Purification of intracellular compartments Visualization of intracellular compartments and proteins 1 Self-assembly of amphiphilic molecules Amphipathic (amphiphilic) molecules hydrophobe hydrophile spontaneously form micelles or bilayers depending on the relative size of the hydrophilic and hydrophobic parts 2 Biological membranes are made of lipids and proteins The fluid mosaic model of cell membranes S. J. Singer and G. L. Nicolson. Science 175(1972):723 The main biological lipids are phospholipides, sphingolipides, glycolipides and cholesterol Biological membranes contain between 25% and 75% proteins (w/w) In cells, 20% (w/w) of proteins are membrane-bound 70% of all eukaryote proteins interact with membranes 3 Fatty acids palmitate oleate acyl chain carboxylic acid group Natural fatty acids contain an even number of carbon atoms: C14->C24 Some bonds may be unsaturated and induce a bend in the structure 4 Phospholipids example of phosphoglycerides ALCOHOL PHOSPHATE G L Y C E R O L FATTY ACID FATTY ACID 5 Phosphoglycerides contain a glycerol, two fatty acids, a phosphate and an alcohol group linked by ester bonds O H3C H2C O C (CH2)14 HC O C (CH2)7 O H3C N+ CH2 H3C CH2 O P O palmitate CH2 CH3 C H C H (CH2)7 CH3 O oleate Oalcohol phosphate glycerol fatty acids phosphatidylcholine 6 Phospholipid diversity derives from many different alcohol moieties... NH3+ H3N+ CH2 CH2 -OOC OH ethanolamine C H CH2 CH2 OH OH CH2 C H H3C choline OH OH H H H OH H HO OH N+ OH OH serine H3C H3C CH2 CH2 H H OH glycerol OH inositol proportion in % Plasma membrane Mitochondria Endoplasmic reticulum Phosphatidylethanolamine Phosphatidylserine Phosphatidylcholine Phosphatidylinositol Sphingomyelin Glycolipids Cholesterol Others 7 4 24 <1 19 7 17 22 35 2 39 0 0 0 3 21 17 5 40 0 5 0 6 30 7 ... associated with many different fatty acids Number of carbon atoms Number of double bonds Laurate Myristate Palmitate Stearate Arachidate Behenate Lignocerate 12 14 16 18 20 22 24 0 0 0 0 0 0 0 Palmitoleate Oleate Linoleate Linolenate Arachidonate 16 18 18 18 20 1 1 2 3 4 hundreds of different phospholipids ! 8 Sphingolipids oleate O C HC CH O H3C N+ H3C HN CH2 H3C alcohol (choline) CH2 O P O CH2 (CH2)7 C H C H C H C H (CH2)12 (CH2)7 CH3 CH3 OH Ophosphate sphingosine sphingomyelin 9 Glycolipids oleate O H HO O H CH2OH H OH O HN C HC CH CH2 (CH2)7 C H C H C H C H (CH2)12 (CH2)7 CH3 CH3 OH H OH H sphingosine polysaccharide (glucose) Glucosylcerebroside and gangliosides For more details concerning ganglioside nomenclature, see http://www.chups.jussieu.fr/polys/biochimie/STbioch/POLY.Chp.10.12.html 10 In biological membranes, the lipid distribution is asymmetric example : plasma membrane Phosphatidylserine Phosphatidylethanolamine Phosphatidylcholine Glycolipids outside inside 0 10 90 100 100 90 10 0 11 Polysaccharides are exposed in the outer leaflet of the plasma membrane 12 Most eukaryote membranes contain cholesterol or ergosterol HO HO Wayne W. LaMorte, Boston University School of Public Health cholesterol ergosterol animal cells fungi cells 13 Cholesterol is essential for the growth and viability of cells in higher organisms The presence of cholesterol and phospholipid diversity are required for the function of many membrane proteins Glutamate transporter GABA transporter Shouffani & Kanner (1990) J Biol Chem 265 : 6002 Steroid hormones derive from cholesterol 14 Lipid mixtures can form microdomains in biological membranes Demixion of lipid mixtures Some microdomains of the plasma membrane called ‘lipid rafts’ seem to be enriched in specific proteins (caveolins), sphingolipids and cholesterol (K. Simons, G van Meer 1988). The existence and physiological function of these structures is still discussed DPPC/Cholesterol 2/1 DPPC : di-palmitoyl phosphatidyl choline GM1 : a ganglioside containing 4 sugars and 1 sialic acid DPPC/Cholesterol/GM1 68/30/2 Lipid monolayer on mica Yuan et Johnston (2000) Biophys. J 79 : 2768 15 Biological membranes and cell compartments Structure of lipid bilayers Structure of membrane proteins Membrane dynamics Purification of intracellular compartments Visualization of intracellular compartments and proteins 16 Interaction of proteins with membranes Integral membrane proteins Solubilized only by using detergents (membrane disruption) Peripheral membrane proteins Solubilized by adding salts or changing the pH (no disruption of the membrane) 17 Membrane protein solubilization by detergents non-ionic detergent ionic detergent 18 The membrane-spanning region of integral membrane proteins is often made of hydrophobic a-helices example : glycophorin A outside 21 hydrophobic amino acids example : b-adrenergic receptor inside 4 nm 19 Transmembrane a-helix can be predicted from the primary sequence Polarity scale Amino acid Phe Met Ile Leu Val Cys Trp Ala Thr Gly Ser Pro Tyr His Gln Asn Glu Lys Asp Arg F M I L V C W A T G S P Y H Q N E K D R Transfert energy (kcal/mole) + 3,7 + 3,4 + 3,1 + 2,8 + 2,6 + 2,0 + 1,9 + 1,6 + 1,2 + 1,0 + 0,6 - 0,2 - 0,7 - 3,0 - 4,1 - 4,8 - 8,2 - 8,8 - 9,2 - 12,3 LSTTEVAMHTTTSSSVSKSYISSQTNDTHK... Score 4,6 2,4 2,4 -2,9 20 Some integral membrane proteins contain several transmembrane segments arranged as a rigid β-barrel Receptor 8 b-strands Lipase 12 b-strands H20 transport 16 b-strands iron transport 22 b-strands In bacteria, mitochondria, chloroplasts 21 In pore-forming toxins, several polypeptides associate to form a large transmembrane domain perfringolysin O (1PFO), a bacterial toxin Plu-MAC/PF ( 2QP2), the core component of the mammalian membrane attack complex (MAC) and perforin (PF) (cf immunology : complement, cytotoxic T cells, natural killer cells) Pore-forming toxins have the ability to switch from a water-soluble form to a membrane-inserted pore 22 form Biological membranes and cell compartments Structure of lipid bilayers Structure of membrane proteins Membrane dynamics Purification of intracellular compartments Visualization of intracellular compartments and proteins 23 Lipids and membrane proteins diffuse laterally in the plane of the membrane Fluorescence Recovery After Photobleaching (FRAP) t r Lateral diffusion coefficient: Lipids Peripheral membrane proteins Free integral membrane proteins Cytoskeleton anchored proteins 1-2 mm2/s 1 mm2/s 0.1-0.5 mm2/s 10-4 mm2/s t = r2/D 24 Membrane dynamics : transport across the lipid bilayer Lipid bilayers are impermeable to ions and polar molecules, but permeable to hydrophobic molecules (moles/sec) Flux permeability = (cm.sec-1) Concentration . Area gradient (cm2) (moles/cm3) Specific membrane proteins carry out the transport of polar molecules ions 25 Facilitated transport Diffusive transport Flux = Permeability . Concentration gradient . Area Facilitated diffusion (Michaelis-Menten kinetics) Flux = Carrier density . Carrier activity . Concentration gradient . Area Carried-mediated diffusion is saturable and mediated by transmembrane proteins (e.g. ion channels) Passive transport carries ions in the direction of the concentration gradient Active transport used ATP hydrolysis energy or another favorable concentration gradient to carry ions against a concentration gradient 26 Ionic channels and transmembrane carriers Binding pockets Selectivity pore Ionic channels and transmembrane carriers transport ions or small polar molecules across membranes in the direction of the concentration gradient 27 Pumps Example : the Na+/K+ ATPase The motive force is provided by ATP hydrolysis 28 Coupled transport (exchangers) Transported molecule The motive force is provided by the favorable concentration gradient of the co-transported ion 29 Example : active glucose transport glucose 2 Na+ 120 mM X? Na+/glucose symporter Transmembrane potential - 60 mV 30 mM carrier pump 30 Conclusion : membrane structure Biological membranes are made of lipids and proteins The lipid bilayer is asymmetrical Membrane proteins are intrinsically or peripherally associated with the lipid bilayer Most intrinsic membrane proteins have transmembrane helices The phospholipids, the sphingolipids, the glycolipids and the cholesterol are the major lipids Lipid mixtures can form subdomains in the biological membranes (lipid rafts) Biological membranes are two-dimensional fluids Biological membranes are dynamic, molecules can cross it thanks to specific proteins Biological membranes are often associated to the cell cytoskeletons The plasma membrane is anchored to the extracellular matrix by adhesion proteins 31 Biological membranes and cell compartments Structure of lipid bilayers Structure of membrane proteins Membrane dynamics Purification of intracellular compartments Visualization of intracellular compartments and proteins 32 Subcellular compartment preparation Cells in culture Cells in tissue Trypsin-EDTA treatment Mechanical shear stress Cells in suspension (106) • Rupture of the plasma membrane Mechanical rupture or plasma membrane solubilization by non-ionic detergents • Separation of the nucleus from the cytoplasm by centrifugation Post-nuclear supernatant • Organelle purification by centrifugation • Separation of organelle membranes and soluble components • Solubilization of organelle membranes using detergents 33 Sedimentation velocity Steady state sedimentation : Friction force + Centrifugal force = 0 f.u DrV0 w2 r f.u = DrV0 w2 r u : particle velocity V0 : particle volume Dr : difference between the density of the particle and that of the surrounding fluid w2 r : relative centrifugal force (often expressed in g); w angular velocity of the rotor ; r distance to the rotor axis S = u/w2r = DrV0/f is the sedimentation coefficient (unit S, svedberg, 1 Svedberg (S) = 10-13 s) For a spherical particle or radius r0, f = 6phr0 (Stocke’s law) h: fluid dynamic viscosity (Pa.s) S = (Drh).(r02/9) Otherwise, f can be determined by measuring D (for instance in DLS experiments), thanks to Einstein formula : f = kBT/D = RT/NAD 34 Sedimentation equilibrium D.dC/dr Steady state : Diffusion flow + Centrifugal flow = 0 C.u(r) D.dC/dr = C.u = C.DrV0 w2 r/f C : particle concentration D : diffusion coefficient V0 : particle volume Dr : difference between the density of the particle and that of the surrounding fluid w2 r : relative centrifugal force (often expressed in g); w angular velocity of bottom the rotor ; r distance to the rotor axis 2 Igor-Bricker sample Absorbance Integrating the differential equation gives C = C0exp[(r2-r02).(DrV0 w2 /2RT)] T=20.0 oC 24,000 RPM v 2=0.73 mL/g meniscus 1 This allows for a precise determination of DrV0 molecular weight of proteins or protein complexes 0 40 45 2 2 r /cm 50 35 Sedimentation equilibrium in a gradient The particles are centrifuged in a density gradient (sucrose, glycerol, Percoll) DrV0 w2 r Buoyancy DrV0 w2 r At steady state : Dr = 0 The position of the particle matches its density This allows for the separation of particles according to their density Step gradients, linear or exponential gradients are used Percoll is a self-forming density gradient particle mix 36 Subcellular compartment fractionation Differential centrifugation sedimentation coefficient (S) = speed (v) acceleration (Dmw2r) proteins ≈ 2-20 S protein complexes ≈ 20 S rRNA ≈ 13, 26, 50 S ribosomes : 50S + 30 S 70S vesicles & virus ≈ 130 S 1 Svedberg (S) = 10-13 s w pelleting factor k = sedimentation coefficient (S) x time (h) ln (rmax/rmin) = w2 X 2,5 1011 rmax Gradient centrifugation rmin sucrose density & osmosis subcellular compartments glycerol density protein complexes density nucleic acids Cesium chloride Percoll® density cells subcellular compartments 37 Gradient centrifugation Velocity sedimentation (separation according to sedimentation velocity S) Fraction collector Equilibrium sedimentation (separation according to buoyant density r) 38 Differential centrifugation Post-nuclear supernatant 1000 x g, 10 min 20 000 x g, 20 min 80 000 x g, 1 hr 150 000 x g, 3 hr Endoplasmic reticulum Golgi apparatus 39 Bovine pulmonary artery endothelial cell labeled with probes to visualize mitochondria, peroxisomes, and the nucleus. Mitochondria were stained with the MitoTracker® Red CMXRos reagent. Peroxisomal labeling was achieved with a primary antibody directed against PMP70, visualized using green-fluorescent Alexa Fluor® 488 goat anti–rabbit IgG. The nucleus was stained with bluefluorescent DAPI. Molecular Probes 40 Immunofluorescence 0. Staining living cells with permeable reagents 1. Cell fixation and permabilization 2. Binding of primary antibodies to specific antigens 3. Visualization using fluorescent secondary antibodies 4. Nucleus visualization using DNA binding fluorophores Thermodynamical balance (1 glucose and 2 Na+ enter together) ln(10)*RT = 1,41 kcal.mole-1 Ne = F = 23 kcal.mole-1.V-1 DG = DGglucose + 2DGNa+ = RT( ln[glucose]int - ln[glucose]ext ) + 2Ne(Vint - Vext) + 2RT( ln[Na+]int - ln[Na+]ext ) = 1.41*( log[glucose]int - log[glucose]ext ) – 2*23*0.06 + 2*1.41*( log[Na+]int - log[Na+]ext ) Transport is possible when DG ≤ 0 and stops when DG = 0 log([glucose]int /[glucose]ext ) ≤ 2*23*0.06/1.41 – 2*log([Na+]int/[Na+]ext ) ≤ 1.96 – 2*log(30/120) ≤ 1.96 + 1.20 = 3.16 Therefore, [glucose]int /[glucose]ext ≤ 1450 42