H 2 O
advertisement

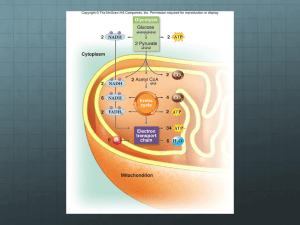
Chapter 7 Biological Oxidation Biological oxidation is the cellular process in which the organic substances release energy (ATP), produce CO2 and H2O through oxidative-reductive reactions. organic substances: carbohydrate, fat and protein 7.1 Principal of Redox Reaction The electron-donating molecule in a oxidation-reduction reaction is called the reducing agent or reductant; the electron-accepting molecule is the oxidizing agent or oxidant: for example: Fe2+ (ferrous) lose -e Fe3+ (ferric) gain +e Redox reaction = reduction-oxidation reaction Several forms of Biological Reduction 1. Gain of electrons 2. Hydrogenation 3. Deoxygenation Several forms of Biological Oxidation 1. Loss of electrons 2. Dehydrogenation 3. Oxygenation Áoxidation-reduction potential ( or redox potential), E : it is a measure of the affinity of a substance for electrons. It decide the loss (or the gain) of electrons. ÁA positive E: the substance has a higher affinity for electrons , accept electrons easily. A negative E: the substance has a lower affinity for electrons , donate electrons easily. E0`, the standard redox potential for a substance :is measured under stander condition(25℃, 1mmol/L reaction substance),at pH7, and is expressed in volts. Section 7.2 Respiration Chain and Oxidative Phosphorylation 7.2.1 Respiratory Chain • Term: A chain in the mitochondria consists of a number of redox carriers for transferring electrons from the substrate to molecular oxygen to form oxygen ion, which combines with protons to form water. Redox carriers including 4 protein complexes 1.Complex I: NADH:ubiquinone oxidoreductase NADH:CoQ oxidoreductase 2.Complex II: Succinate dehydrogenase 3.Complex III: cytochrome bc1 (ubiquinone Cyt c oxidoreductase) 4.Complex IV: cytochrome oxidase Complex I (NADH:ubiquinone oxidoreductase) • Function: transfer electrons from NADH to CoQ • Components: NADH dehydrogenase (FMN) Iron-sulfur proteins (Fe-S) complex Ⅰ NADH→ FMN; Fe-SN-1a,b; Fe-SN-4; Fe-SN-3; Fe-SN-2 →CoQ R=H: NAD+; R=H2PO3:NADP+ 1. NAD(P)+: Nicotinamide Adenine Dinucleotide Phosphate) Oxidation of NADH is a 2electron(2e), 2-proton(2H) reaction NAD+ or NADP+ NADH or NADPH 2. FMN can transfer 1 or 2 hydride ions each time FMN: flavin mononucleotide Accepts 1 H+ and 1 eto form semiquinone = stable free radical Accepts 2 H+ and 2 eto give fully reduced form 3. Iron-sulfur clusters (Fe-S) transfers 1-electron at a time, without proton involved Fe3++e- Fe2+ 4.Ubiquinone (CoQ) is lipid-soluble, not a component of complex Ⅰ, can transfer 1 or 2 hydride ions each time. Function: transfer electrons and protons from complex Ⅰ,Ⅱto complex Ⅲ. NADH+H+ NAD+ FMN Reduced Fe-S Q FMNH2 Oxidized Fe-S QH2 Matrix Intermembrane space Complex II: Succinate dehydrogenase (Succinate: CoQ oxidoreductase) • Function: transfer electrons from succinate to CoQ • Components: Succinate dehydrogenase (FAD, Fe-S) Cytochrome b560 Complex Ⅱ Succinate→ Fe-S1; b560; FAD; Fe-S2 ; Fe-S3 →CoQ Cytochromes a, b, c are heme proteins, their heme irons participate redox reactions of e- transport. Fe3++e- Fe2+ Intermembrane space Matrix Succinate Complex III: cytochrome bc1 (ubiquinone Cyt c oxidoreductase) • Function: transfer electrons from CoQ to cytochrome c • Components: iron-sulfur protein cytochrome b(b562, b566) cytochrome c1 complex Ⅲ QH2→ b562; b566; Fe-S; c1 →Cyt c Cytochrome c is soluble, which will transfer electrons to complex Ⅳ Intermembrane space Matrix Complex IV: cytochrome oxidase • Function: transfer electrons from Cyt c to molecule oxygen, the final electron acceptor. • Components: cytochrome aa3 copper ion (Cu2+) Cu2+ + eCu+ Complex IV Cyt c → CuA→a→a3→CuB → O2 Cytochrome c Coenzyme Q ubiquinone/ol Sequence of respiratory chain Principles: • e- tend to flow from a redox pair with a lower E°to one with a higher E° • In the e--transport chain, e--carriers are arranged in order of increasing redox potential, making possible the gradual release of energy stored in NADH, FADH2 Redox potential redox pair E0 There are two respiratory chains • NADH respiratory chain NADH Complex Ⅰ CoQ Complex Ⅲ cytochrome c Complex Ⅳ O2 • Succinate (FADH2) respiratory chain Succinate ComplexⅡ CoQ ComplexⅢ cytochrome c ComplexⅣ O2 NADH respiration chain FADH2 respiration chain 7.2.2 Oxidative Phosphorylation • The oxidation of organic nutritions produces the energy-rich molecules, NADH and FADH2. • The oxidation of NADH or FADH2 in mitochondrial is the electron transferring through respiration chain. • The free energy produced in electron transferring supports the phosphorylation of ADP to form ATP. • The oxidation of NADH or FADH2 and the formation of ATP are coupled process, called Oxidation Phosphorylation. The Chemiosmotic Theory • The free energy of electron transport is conserved by pumping protons from the mitochondrial matrix to the intermembrane space so as to create an electrochemical H+ gradient across the inner mitochondrial membrane. The electrochemical potential of this gradient is harnessed to synthesize ATP. Peter Mitchell Electrochemical H+ gradient (Proton-motive force) 2 components involved 1. Chemical potential energy due to difference in [H+] in two regions separated by a membrane 2. Electrical potential energy that results from the separation of charge when a proton moves across the membrane without a electron. Complex I: 4 H+ expelled per e--pair transferred to Q Complex III: 4 H+ expelled per e--pair transferred to Cyt c Complex IV: 2e- + 2 H+ from matrix convert ½ O2 to H2O; 2 further H+ expelled from matrix Proton pumping: Reductiondependent conformational switch of an e--transport complex Conformation 1 (high affinity for H+) Conformation 2 (low affinity for H+). ATP Synthase Intermembrane space Inner (ab2c9-12) Membrane Matrix C ring (α3β3γδε) β-subunit take up ADP and Pi to form ATP ADP + Pi ATP Each of 3 b-subunits contains an active site F1: multisubunit complex that catalyzes ATP synthesis F0 = proton-conducting transmembrane unit When protons flow back through F0 channel, γ-subunit is rotated by the rotation of c ring, then the conformations of β-subunits are changed, this lead to the synthesis and release of ATP. To form a ATP need 3 protons flow into matrix. H+ flow β-subunit has three conformations:T (tight), L (loose), O (open) Translocation of ATP , ADP and Pi. H+ ADP3- ATP4- H2PO4- H+ Intermembrane 胞液侧 space F 0 基质侧 Matrix F1 H2PO4- H+ ATP4ADP3H+ P/O ratios • P/O ratio is the rate of phosphate incorporated into ATP to atoms of O2 utilized. It measure the number of ATP molecules formed per two electrons transfer through the respiratory chain. • NADH respiratory chain : 2.5, • FADH2 respiratory chain: 1.5 • During two electrons transfer through NADH respiratory chain, ten protons are pumped out of the matrix. • To synthesis and translocation an ATP, four protons are needed. • So, two electrons transport can result in 2.5 ATP. • To succinate respiratory chain , two electrons transport can result in 1.5 ATP. Regulation of Oxidative Phosphorylation • 1.PMF (proton motive force) regulate the electron transport. higher PMF lower rate of transport • 2.ADP concentration resting condition: energy demanded is low, ADP concentration is low, the speed of Oxidative Phosphorytion is low. active condition: the speed is high. Inhibitor of Oxidative Phosphorylation • 1.Inhibitor of electron transport Succinate Antimycin A Cyanide, Azide Carbon Monoxide × × × Retonone Amytal • 2.Uncoupling agents uncoupling protein (in brown adipose tissue), 2,4-dinitrophenol, Pentachlorophenol heat H+ Intermenbran space Ⅰ Ⅱ H Cyt c uncoupling protein F Q 0 Ⅲ Ⅳ F1 Matrix + H+ H+ ADP+Pi ATP 2,4-dinitrophnol 3.Oligomycin bonds at the connection of F0 and F1, inhibit the function of ATP synthase. Intermembrane space Matrix Oligomycin C ring Succinate Ⅱ Retonone Amytal Ⅰ Antimycin A × Ⅲ × × Oligomycin Uncoupling agent Ⅴ × × Ⅳ ATP and other Energy-rich compounts ATP has two energy-rich phosphoric acid anhydride bonds, the hydrolysis of each bond release more energy than simple phosphate esters. NH 2 N N OH O= P OH OH OH O ~p O ~p OH N N OCH2 O H OH H H H OH OH AMP ADP ATP Some Energy-rich compounds Structure Exemple creatine phosphate phosphoenolpyruvate acetyl phosphate Acetyl CoA ΔGº’ • The hydrolysis of energy-rich bond: ΔGº’ = -5~-15kcal/mol • The compounds with energy-rich bond are high-energy compounds. • The hydrolysis of low-energy bond: ΔGº’ = -1~-3kcal/mol • The compounds with low energy bond are low-energy compounds. Transport of high-energy bond energies • 1.Substrate level phosphorylation Glycerate 1,3-biphosphate + ADP Glycerate 3-phosphate +ATP ΔGº’ = -4.5kcal/mol Phosphoenolpyruvate +ADP Pyruvate + ATP ΔGº’ = -7.5kcal/mol 2.ATP is the center of energy producing and utilizing. ATP Oxidative Phosphorylation Energy utilization Substrate level phosphorylation ~P ~P ADP 3.Other nucleoside triphosphates are involved in energy transport. • GTP: gluconeogenesis protein synthesis • UTP: glycogen • CTP: lipid synthesis 4.Transport of the terminal phosphate bond of ATP to the other nucleoside • Function of nucleoside diphosphate kinase ATP + UDP ATP + CDP ATP + GDP ADP + UTP ADP + CTP ADP + GTP • Function of adenylate kinase ADP + ADP ATP + AMP 7.3 Energy from cytosolic NADH • A mitochondrial NADH produce 2.5 ATP • A cytosolic NADH must be transported into mitochondrial for oxidation by two methods. Glycerol phosphate shuttle 1.5 ATP Malate aspartate shuttle 2.5 ATP Glycerol phosphate shuttle CH2OH CH2OH Electron chain NADH+H+ Glycerol phosphate dehydrogenase NAD+ C=O C=O CH2O- Pi CH2O- Pi dihydroxyacetone phosphate dihydroxyacetone phosphate CH2OH CH2OH CHOH CHOH CH2O- Pi CH2O- Pi Glycerol phosphate Glycerol phosphate Intermembran space FADH2 FAD Glycerol phosphate dehydrogenase Inner menbran Malate aspartate shuttle O Aspartate -OOC-CH2-C-COO- H 3N Malate α-ketoglutarate carrier - + OOC-CH2-C-COO H oxaloacetate - Aspartate oxaloacetate Glutamate Glutamate Electron chain NADH +H+ NADH +H+ NAD+ α-ketoglutarate α-ketoglutarate OH NAD+ -OOC-CH2-C-COOH Malate cytosol Glutamate-aspartate carrier inner mitochondrial membran Malate matrix 7.4 Other Biological Oxidations • Monoxygenases dioxygenase --add 2 atoms of O2 oxygenase to organic compounds. monoxygenase (mixed-function oxidase, hydroxylase) --adds 1 oxygen atom to organic compounds as a hydroxyl group. RH + NADPH + H+ + O2 ROH + NADP+ + H2O The chief compounds of monoxygenase: Cyt b5, Cyt P450, Cyt P450 reductase(FAD,FMN) Free Radical Scavenging Enzymes Free Radical: the groups with an unpaired electron. (such as O2﹣、 H2O2、•OH) 1.Superoxide dismutases(SODs) 2O2﹣+ 2H+ SOD H2O2 + O2 peroxidase H2O + O2 • 2.Glutathione peroxidase H2O2 (ROOH) 2G –SH Glutathion e Glutathione reductase peroxidase H2O (ROH+H2O) NADP+ G –S – S – G NADPH+H+ • 3.Catalase (in peroxisomes) 2H2O2 catalase 2H2O + O2 summary