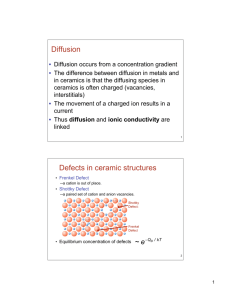
Defects in oxides
advertisement
Lattice defects in oxides. Correlations between defects, properties and crystal structures Defects in oxides Lattice or point defects: Extended defects: • Vacancies (oxygen, cation) • Crystallographic shear • Interstitial ions • Dislocations • Foreign atoms at regular sites (doping/solid solutions) • Grain boundaries • Defect pairs and clusters • Electronic defects (free electrons and holes) Defect chemistry. Lattice defect can be treated as chemical entities (energy of formation) using defect reactions (mass-action law, equilibrium constant). Kroger-Vink notation for defects. Defect charge is referred to the perfect crystal. Binary oxides (ZnO) Ternary oxides (ZnAl2O4) Cation vacancy VZn'' Oxygen vacancy VO Interstitial cation Zni Interstitial oxygen Oi'' Foreign ion (donor) AlZn Foreign ion (acceptor) Antisite defects ' AlZn ZnAl ' NaZn Defects in oxides Rules for defect reactions: • Site relation. The number of sites must be in the correct proportion (MaXb: M/X = a/b). • Sites can be created or destroyed taking into account the site relation. • Mass balance. • Electroneutrality condition. 1eV = 96.5 kJ/mol Frenkel disorder AgBr ' AgAg Vi Agi VAg Schottky disorder '' MgMg OO MgO VMg VO Mgsurf Osurf '' zero MgO VMg VO Defects and entropy N atoms arranged at (N+n) sites with n vacancies G G0 nHv T Sconf nSvibr N n ! Sconf k B ln W k B ln N!n! Hv 0; Sconf 0; Thermodynamic probability for distinguishable particles No! W no !n1!n2!...nr ! - Svibr 0 N0: total number of atoms ni: number of atoms on the i-th energy state Defects and nonstoichiometry in binary oxides Oxygen nonstoichiometry (TiO2, CeO2, Nb2O5, V2O5) ' 1 OO CeO 2 VO 2CeCe O2 2 ' CeCe Ce3 1 Oo CeO 2 VO 2e ' O2 2 Metal nonstoichiometry (FeO, NiO, MnO) 1 O2 FeO OO VFe'' 2 Fe Fe 2 FeFe Fe3 Fe1-yO 1 O2 FeO OO VFe'' 2h 2 Defects and nonstoichiometry in binary oxides 1 Oo CeO 2 VO 2e ' O2 2 K1 [VO ]n2 pO1/22 CeO2-x x: fraction of vacant sites in CeO2-x K1' [VO ]3 pO1/22 x3 pO1/22 x pO12 / 6 1/ n O2 x p n = 6: doubly ionized vacancies n = 4: singly ionized vacancies n = 2: neutral vacancies Isolated defects In general: n ≤6 for isolated defects or defect complexes G O2 O2 O02 RT ln pO2 nRT ln x - log x in CeO2-x Defect ordering Formation of subphases O Electroneutrality Defect complexes V 12 x n e' -GO2 (kcal/mol) n 2 VO Equilibrium constant Defects and nonstoichiometry in binary oxides. Formation of shear planes Ordering of defects and formation of superstructures is observed for large deviations from stoichiometry (TiO2-δ, Nb2O5-δ, WO3-δ, ReO3-δ, etc.). Elimination of oxygen vacancies by formation of metal-rich shear planes is a common mechanisms (crystallographic shear). Formation of shear planes in ReO3 (left) and WO3 (right) by elimination of oxygen vacancies Lattice defects and nonstoichiometry in perovskites BaTiO3 Partial Schottky disorder (TiO2–rich side) Partial Schottky disorder (BaO-rich side) Full Schottky disorder Oxygen nonstoichiometry 1eV = 96.5 kJ/mol Lattice defects and nonstoichiometry in perovskites Ti-rich Ba-rich BaTiO3 Lattice defects, nonstoichiometry and phase transitions in perovskites Cubic (paraelectric) – tetragonal (ferroelectric) phase transition in BaTiO3 Enthalpy of transition 1200°C Ti-rich Transition temperature Ba-rich Ba-rich 1320°C Ti-rich Lattice defects and electrical conductivity in perovskites Electrical conductivity Z: numero di cariche; e: carica dell’elettrone; : mobilità c: concentrazione zi e i ci n e'; p h (1)m = -1/6 BaTiO3 (2)m = -1/4 (1) At low p(O2) << p0(O2) 1 OO O2 VO 2e ' 2 K1 VO n2 pO1/22 n 2 VO n pO12 / 6 (2a) At intermediate p(O2) and R = Ba/Ti < 1 V V aR '' Ba O n pO12 / 4 (2b) At intermediate p(O2) with R = 1 and acceptor impurities n-type p0(O2) n=p Reduction (1): H2-3 eV Oxidation (3): H1 eV p-type A 2V O n pO12 / 4 (3) At high p(O2) p(O2) > p0(O2) 1 O2 VO OO 2h 2 K 2 p 2 VO 1 pO12 / 2 p VO pO12 / 4 Doping of perovskites: controlling defect nature and concentration Acceptor doping: the substitutional impurity has a lower charge than the regular and bring less oxygen into the lattice Fe2O3 2BaO BaTiO 3 2BaBa 2FeTi' VO 5OO 1 O2 Fe2O3 2 BaO BaTiO 3 2 Ba Ba 2 FeTi' 2h 6OO 2 ' Na2O 2TiO2 BaTiO 3 2NaBa 2TiTi VO 5OO Donor doping: the substitutional impurity has a higher charge than the regular and bring more oxygen into the lattice 1 La2O3 2TiO2 O2 2 LaBa 2TiTi 2e ' 6OO 2 BaTiO3 '' La2O3 3TiO2 2LaBa VBa 2TiTi 9OO 1 Nb2O5 2 BaO BaTiO 3 O2 2 NbTi 2 Ba Ba 2e ' 6OO 2 '' Nb2O5 BaO BaTiO 3 2NbTi VBa BaBa 6OO BaTiO3 Doping of perovskites: influence of doping on electron conductivity Donor doped compounds: • Black colour; • Good conductivity (>10-2 S/cm) even at RT; • Some show metallic conduction (103 S/cm, La:SrTiO3); 1 La2O3 2TiO2 BaTiO 3 O2 2 LaBa 2TiTi 2e ' 6OO 2 La2O3 3TiO2 BaTiO 3 2LaBa VBa'' 2TiTi 9OO 1 OO O2 VO 2e ' 2 La:BaTiO3 1200°C p0(O2) Doping of perovskites: influence of doping on electron conductivity Acceptor doped compounds • Light colour; • Good conductivity at high temperature • Many are insulators at RT; • Can be fired in reduced atmosphere retaining their dielectric properties. p0(O2) Mn2O3 2BaO BaTiO 3 2BaBa 2MnTi' VO 5OO 1 O2 Mn 2O3 2 BaO BaTiO 3 2 Ba Ba 2MnTi' 2h 6OO 2 Doping of perovskites: from isolated defect to oxygen vacancy ordering and formation of layered structures Due to the high dielectric constant (20-1000) and structural stability, perovskites can accomodate a large concentration of foreign aliovalent impurities (good solvent) and related charge compensating defects (cation or oxygen vacancies). The simple model of randomly distributed isolated defects (no association) holds up to high dopant concentration (few at.% for acceptors, 10 at.% for donors). At higher dopant concentration, ordering of defects, formation of shear planes and layered structures is observed. Fe2O3 2SrO SrTiO 3 2SrSr 2FeTi' VO 5OO SrFexTi1 xO3 x / 2 ; FeTi' 2 VO x = 0: SrTiO3; x = 1: Sr2Fe2O5 At T < 700°C, oxygen vacancy ordering occurs in Sr2Fe2O5 (brownmillerite structure). For intermediate compositions, intergrowth of perovskite blocks and brownmillerite layer with general formula AnBnO3n-1. Perovskites doped with high concentration of acceptor impurities (10-20 at.%) shows high ionic (oxygen) and electronic (holes related to transition metals Ti, Fe, Co, Nb) conductivity. Application as mixed conductors in electrochemical devices. ABO3 A2B2O 5 Sr2Fe2O5 A4B4O11 Doping of perovskites: from isolated defect to oxygen vacancy ordering and formation of layered structures La1-xSrxFeO3 with 0 < x <0.25 can be accurately described as an acceptor-doped perovskite with randomly distributed defects At low p(O2): 2SrO 2Fe2O3 LaFeO 3 2SrLa' 2FeFe VO 5OO 1 OO O2 VO 2e ' 2 Fe2+ p-type n-type At high p(O2) 1 O2 VO OO 2h 2 Fe4+ p-type n-type Doping of perovskites: excess oxygen, shear planes and layered structures Reducing atmosphere, black conducting ceramics (up to 103 Scm-1) , random distribution of defects, x up to 0.3 x La2O3 (1 x) SrO TiO2 2 x La x Sr1 xTiO3 O2 2 Oxidizing atmosphere, less conducting, O excess accomodated by formation of shear planes when x > 0.17, by small isolated defects when x<0.17 x La2O3 (1 x) SrO TiO2 2 Lax Sr1 xTiO3 δ = x/2 x = 0, δ = 0 : SrTiO3 x = 1, δ = 0.5 : La2Ti2O7 cubic distorted orthorhombic The structure can be described as the intergrowth of perovskite layers (SrTiO3) and La2Ti2O7 layers. A layered perovskite with general formula: La4Srn-4TinO3n+2 SrTiO3 La2Ti2O7 Layered perovskites A typical example: Ruddlesden-Popper phases SrO(SrTiO3)n or Srn+1TinO3n+1 Sr3Ti2O7 P SrO P SrO SrTiO3+Sr3Ti2O7 P Sr3Ti2O7 (n = 2) Aurivillius compounds: ((Bi2O2)2+(Bim-1TimO3m+1)2-) Ferroelectric & piezoelectric materials with high TC