Prof. McKittrick's lecture notes
advertisement

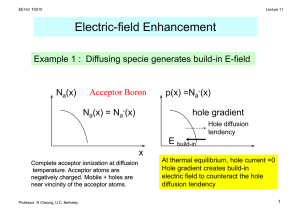
Diffusion
• Diffusion occurs from a concentration gradient
• The difference between diffusion in metals and
in ceramics is that the diffusing species in
ceramics is often charged (vacancies,
interstitials)
• The movement of a charged ion results in a
current
• Thus diffusion and ionic conductivity are
linked
1
Defects in ceramic structures
• Frenkel Defect
--a cation is out of place.
• Shottky Defect
--a paired set of cation and anion vacancies.
Shottky
Defect:
Frenkel
Defect
• Equilibrium concentration of defects
~ e !QD / kT
2
1
Schottky and Frenkel defects
Schottky defects in NaCl
Both cation and anion are
missing from their regular
lattice sites
At room temperature, 1 in
1015 sites are vacant
200 kJ/mole (2.7 eV) creation
energy
Cation Frenkel defects in
AgCl
Cation displaced from
regular lattice site onto
interstitial site
150 kJ/mole (1.6 eV)
creation energy
3
Impurities
• Impurities must also satisfy charge balance = Electroneutrality
• Ex: NaCl
Na +
Cl -
• Substitutional cation impurity
cation
vacancy
Ca 2+
Na +
Na +
initial geometry
Ca 2+ impurity
• Substitutional anion impurity
O2-
initial geometry
Cl Cl 2O impurity
Ca 2+
resulting geometry
anion vacancy
resulting geometry
4
2
Crystalline point defects
• If cationic impurities are introduced into a solid and the
dopant does not have the same valence as the cation
it is replacing, extrinsic defects will be introduced
– Ca2+, Y3+ in ZrO2 have anion vacancies
– Ca2+ or Cd2+ in NaCl creates cation vacancies
• Real crystals contain both intrinsic and extrinsic
defects
• The dominate defect type depends on temperature
and doping level
– Typically
• High temperatures
– intrinsic
• Low temperatures
– extrinsic defects
5
Kröger-Vink notation
• Standard notation for defects in ionic crystals
• Composed of 3 parts
• Main body identifies the defect
– V = vacancy
– M = metal
– X = non-metal
• Subscript denotes site the defect occupies
– i = interstitial
– x = non-metal
– M= cation site
• Superscript identifies the effective charge
–
–
• = positive charge
' = negative charge
6
3
Examples
Consider MgO
''
VMg
A vacancy on the Mg site
It has a double negative charge since Mg is 2+
VO••
A vacancy on the O site
It has a double positive charge since O is 2-
Ali•••
An Al interstitial
It has a triple positive charge since it is 3+
•
AlMg
'
LiMg
An Al on a Mg site
It has a positive charge since Al is 3+ and Mg is 2+
An L on a Mg site
It has a negative charge since Li is 1+ and Mg is 2+
7
Defection associations and
concentrations
Concentrations given in brackets:
•
[AlMg
]
''
[VMg
]
[e '] = n = concentration of electrons
! = p = concentration of holes
[h]
8
4
Defect reactions
Reactions occur for defects, just like other chemical
species in the lattice
Consider Shottky defects in MX (M=cation, X=anion)
There is a random distribution of cation and anion vacancies
VM' +VX• ! null
KS = equilibrium constant = [VM' ][VX• ]
From the definition of the equilibrium constant:
1
# !"gs &
# !"hs + T "ss &
# "s &
# !"hs &
# !"hs &
K s = exp %
= exp %
= exp % s ( exp %
) exp %
(
(
(
(
kT
$ kT '
$
'
$ k '
$ kT '
$ kT '
9
Defect reactions
# !"gs &
(
$ kT '
KS = equilibrium constant = [VM' ][VX• ] = exp %
When these are the only defects present, then
# !"gs &
[VM' ] = [VX• ] = exp %
(
$ 2kT '
Frenkel defects
MM ! VM' + M i•
K F = [VM' ][M i• ]
Electronic defects
e '+ h! ! null
! = np
K e = [e '][h]
10
5
Rules for defect reactions
• These rules must be satisfied:
– Mass conservation
• Not creating or destroying matter!
– Electroneutrality
• The + and - charges must be balanced on each side of
reaction equation
– Site ratio conservation
• Different crystal structures are not created
ai Ai ! bi Bi
k=
" [B ]bi
i
i
" [Ai ]ai
i
% #$G (
= exp '
& kT *)
11
Oxidization and reduction
1/
M O M O M O
2O 2
(g)
2e
O M O M O M
M O M O M O
2h
Oxidation - generate holes
Reduction - generate electrons
O M O M O M
e CB
M (g)
VO•
VO••
VM''
VM'
e
VB
12
6
Oxidation and reduction
1
O2 (g) +VO•• ! OO + 2h!
2
# !"gO &
p2
KO = •• 1/2 = KOo exp %
(
[VO ]pO2
$ kT '
1
OO ! VO•• + 2e '+ O2 (g)
2
# !"gR &
1/2
K R = [VO•• ]n 2 pO2
= K Ro exp %
(
$ kT '
13
Examples
1. Sodium tungstate bronze
NaxWO3 x: 0.32-0.93
'
perovskite with VNa
n-type for x < 0.25
Metallic conductivity for x > 0.25
2. Ce3S4
Ce2.67S4 ρ ~ 10-3 Ω-cm
Ce3S4 ρ ~ 109 Ω-cm
3. BaTiO3 heated and quenched in H2
BaTiO3-x good semiconductor
Ti4+→Ti3+ + h•
4. ZnO
sintering rate increases as pO2 decreases
formation of Zni
14
7
Impurity induced, ion compensated
There is no such thing as a 'pure' material
Can get 99.9999% pure (4 9's, Alfa Aesar, e.g.)
Concentration of impurities is 100 ppm or 10-4
Consider adding CdCl2 to NaCl
Assume Cd sits on Na site (not interstitial, too large)
Cd is 2+, for charge balance must form Na vacancies or
Cl interstitials (unlikely)
2NaCl
•
'
CdCl2 !!!
" Cd Na
+VNa
+ 2ClCl
ZrO2
CaO !!!
" Ca +V + OO
''
Zr
••
O
Na1-2xCdxCl
Zr1-xCaxO2(1-x)
15
Frenkel defects
M
X
M
X
M
X
M
X
X
X
M
M
M
X
M
X
M
X
X
M
AgBr, CaF2
M
N = number of normal sites
N* = number of interstitial sites
" !Q %
nF = (NN * )1/2 exp $ F ' = number of Frenkel painr
# 2kT &
16
8
Assumptions
• Only have one type of predominate defect
– Schottky or Frenkel
• Assume a dilute solution
– Neglect interactions between defects
• Constant volume
• Energy for defect formation independent
of T
17
Diffusion in lightly doped NaCl
Consider adding CdCl2 to NaCl
2NaCl
•
'
CdCl2 !!!
" Cd Na
+VNa
+ 2ClCl
The Na diffusion coefficient is
DNa
% #$GV * (
Na
= [V ]!" exp '
*
'& kT *)
'
Na
2
ΔGVNa* is the energy for migration of free vacancies
At low temperatures, extrinsic behavior observed
$ #S * '
$ *#HNa * '
DNa = [CdCl2 ]!" 2 exp & Na ) exp &
)
% k (
% kT (
18
9
At high temperatures, there are additional vacancies from
Schottky defects that swamp the effect of the impurity
ln D (cm2/sec)
% #$GNa * (
% #$SNa * (
% #$HNa * (
% #$sS (
% #$HS (
'
2
DNa = [VNa
]!" 2 exp '
=
!"
exp
exp
exp
exp
*
'
*
'
*
' 2k *
' 2kT *
&
)
&
)
& kT )
& k )
& kT )
-1/
k(ΔHNa
*
+ 1/2Δhs)
-1/
high T
intrinsic
k(ΔHNa
*)
low T
extrinsic
1/T
19
Diffusion in cation-deficient oxides
The transition metal oxides are typically cation deficient
Ni1-xO, Co1-xO, Mn1-xO, Fe1-xO
x↑
3 x 10-4
10-2 at 1300˚C
Can get up to x = 0.15, then
form F2O3
Consider Co1-xO
1
O (g) = OO +VCo
2 2
K1 = [VCo ]aO!1/2
2
'
VCo = VCo
+ h!
K2 =
'
''
VCo
= VCo
+ h!
K3 =
'
Co
[V ]p
[VCo ]
''
[VCo
]p
'
Co
[V ]
'
''
x = [VCo ] + [VCo
] + [VCo
]
electrical conductivity is ptype
20
10
Diffusion in highly doped oxide - cubic
stabilized ZrO2
''
2
CaO !!!
" CaZr
+VO•• + OO
ZrO
Usually 8-15% added
Brouwer approximation:
defect
clustering
cubic +
tetragonal
single cubic
phase
''
[CaZr
] = [VO•• ]
Large concentration
of oxygen vacancies
compared with most
oxides
ΔG* = 1 eV (small)
Ca1-xZrxO2(1-x) fast ion
conductor
21
Electrical conductivity
• Conductivity values range over 25 orders of magnitude
– Most insulating LiF (band gap > 12 eV)
– Superconductors (no band gap)
• Electrical conductivity arises from
– Movement of charged ions
• Ionic conductivity
– Sensors, electrochemical pumps, solid electrolytes in fuel cells, high T battery systems
– Movement of electrons
• Measured electrical conductivity
– From both ions and electrons
– σtotal = σelec + σion
– ti = transference number = σi/σtotal
• If telec > tion
• If tion > telec
electronic conductor
ionic conductor
• Oxides that are easily reduced are n-type semiconductors
– e.g. TiO2, SnO2, ZnO, BaTiO3
• Oxides that are easily oxidized are p-type semiconductors
– e.g. transition metal monoxides (NiO, FeO, CoO)
22
11
(Ω-cm)-1
IONIC CONDUCTORS
ELECTRON CONDUCTORS
!
YBa2Cu3O7-x
106
metallic
100
Na/S battery
Na β-Al2 O 3
Oxygen sensor
ZrO2-Y2O3 (1000˚C) fast ion
Li2 O-LiCl-B2O3
conductor
(glass, 300˚C)
KxPb1-xF1.75
Primary battery
fluorine ion
LaF3, EuF2
specific electrode
NaCl
RuO2 (thick films)
TiO
LaNiO3 (fuel cell electrode)
SnO2•In2 O 3 (transparent elect.)
SrTiO3 (photoelectrode)
V2O 3 •P2O3 (glass)
semiconducting
TiO2-x (oxygen sensor)
10-6
solid
electrolyte
TiO2
10-12
insulator
passivation on
Si devices
ZnO (varistor)
insulating
Al2O3 (substrate)
SiO2
10-18
23
Mobility
Mobility =
velocity
driving force
chemical, electric field, mechanical
In a chemical gradient, the absolute mobility given by
Bi =
velocity (cm / sec) v i
= =
force (ergs / cm)
Fi
vi
) 1 # "µ!
i
!+
%
N
"x
+* A $
µ! i (ergs/mole)
&,
( . note: this is the chemical potential, not
' .- the electrical mobility
The chemical mobility = Bi' = Bi/NA
24
12
Mobility and diffusivity
Ji = civi = ciBiFi
Ji = !
1 # "µ! i &
%
( Bc
NA $ "x ' i i
For an ideal solution,
µ! i = µo + RT ln ai = µo + RT ln ! i c i " µo + RT ln c i
# 1 & dc
d µ! i
= RT % ( i
dx
$ c i ' dx
Ji = )
dc
1 # RT dc i &
RT dc i
Bc = )
B
= )Di i
NA %$ c i dx (' i i
NA i dx
dx
Di = kTBi
Nernst-Einstein relation
25
Instead of using a chemical potential (hard to measure), put
these expressions in terms of an electric field
d!
= zi eE
dx
"D %
z ec D E
Ji = c i Bi Fi = c i $ i ' (zi eE) = i i i
kT
# kT &
Fi (electrical) = zi e
Ji = c i v i =
zi ec i Di E
kT
zi eDi
E
kT
v
z eD
z FB
µi = i = i i = zi eBi = i i = zi FBi'
E
kT
NA
vi =
F = Faraday's constant =96,500
C/mole = eNA
relating electronic mobility with chemical mobility
26
13
Ionic conductivity
! i = zi e µi c i
µi =
zi2e 2Di c i
!i =
kT
zi eDi
kT
Usually written in (Ω-cm)-1 or S-m-1
where S = Ω-1
e = 1.6 x 10-19 C
D in cm2/sec
c in #/cm3
k in ergs (107ergs = J)
Conductivity depends on
carrier concentration
mobility of carrier
temperature
At room temperature
not many defects
mobility low
27
Diffusion and electrical conductivity
measurements
• Diffusion of a radioactive tracer element Na
was measured
• The electrical conductivity was measured
D
tracer
Difference is ~ 2 x 1011
cm2/sec
conductivity
1/T
28
14
The electrochemical potential
• Gradients in chemical potential (concentration)
and electric field mobilize defects
• Even in the absence of an external field,
internal electric fields are present
– Non uniform distribution of space charge
• Driving force for mass transport is the
electrochemical potential (η) instead of just the
chemical potential
!i = µi + zi "F
F = Faraday's constant = eNA
= 96,500 C/mole
29
The force on the particle, Fi, is the negative gradient of ηi
Fi = !
Ji =
1 # d "i &
NA %$ dx ('
!c i Bi # d"i & !c i Bi # d µ! i
d) &
=
+ zi F
%
(
%
(
NA $ dx '
NA $ dx
dx '
Even a modest electrical field can offset the effect
of the concentration gradient in the opposite
direction
30
15
Ambipolar diffusion
• Coupled transport of different charged species
• Ionic crystals must maintain charge neutrality
– Long range charge separation must be avoided
– Charge species are coupled
• Effect of slowing down faster diffusing species and
speeding up slower diffusing species
• Both diffuse with a common diffusivity
– Chemical or ambipolar diffusion coefficient D!
31
Consider MgO
''
VMg
+VO•• ! null
''
KS = [VMg
][VO•• ]
e '+ h• ! null
K i = np
1
OO ! VO•• + 2e '+ O2 (g)
2
KO = VO•• n 2 pO1/2
2
The flux of oxygen vacancies must be matched by an
equivalent charge flux of electrons outward, holes inward
µe > µh
2JVO = Je
Using the ambipolar diffusion coefficient:
" dc %
VO
'
JV •• = !D! $
O
$# dx '&
" dn %
and Je ' = !D! $ '
# dx &
32
16
~
How does D depend on DVO and De?
Rewrite Fick's first law in terms of ηi acting on the 2 defects
separately, equating the fluxes to solve for the internal field.
2JV •• = !
O
Je '
2cV DV $ "µ!V
"# '
O
O
O
+ 2F )
&
RT &% "x
"x )(
nD
=! e
RT
$ "µ! e
"# '
& "x ! F "x )
%
(
flux is raised by internal field
( µ! = µ!
o
+ RT ln c
)
flux is lowered by internal field
Then, rewriting the 2 expressions to get ∂φ/∂x
D
!" RT (
=
e
!x
JV ••
O
F
# DV
O
!cV
) !x
O
De + 2DV
"
!cV %
!n
=2 O'
$ the concentration gradients,
!x
!x '
$
$# n = 2cV
'&
O
O
$ 3De DV ' !cV
O
O
= #&
)
&% De + 2DV )( !x
O
D! =
3De DV
O
De + 2DV
O
33
~
If De >> DVO, the D = 3DVO
Ambipolar diffusion rate is controlled by the slower species
Ambipolar coupling causes rate to be enhanced by 3X
~
If DVO>>De, then D = 1.5 D
Slower species is rate controlling
Ambipolar coupling increases effective diffusion coefficient
The ambipolar diffusion coefficient is greater than that
of the slower defect, due to charge-coupling to the
faster one
34
17