Formation of Nano-Porous TiO 2 Layer via Anodization in Molten Salts
advertisement
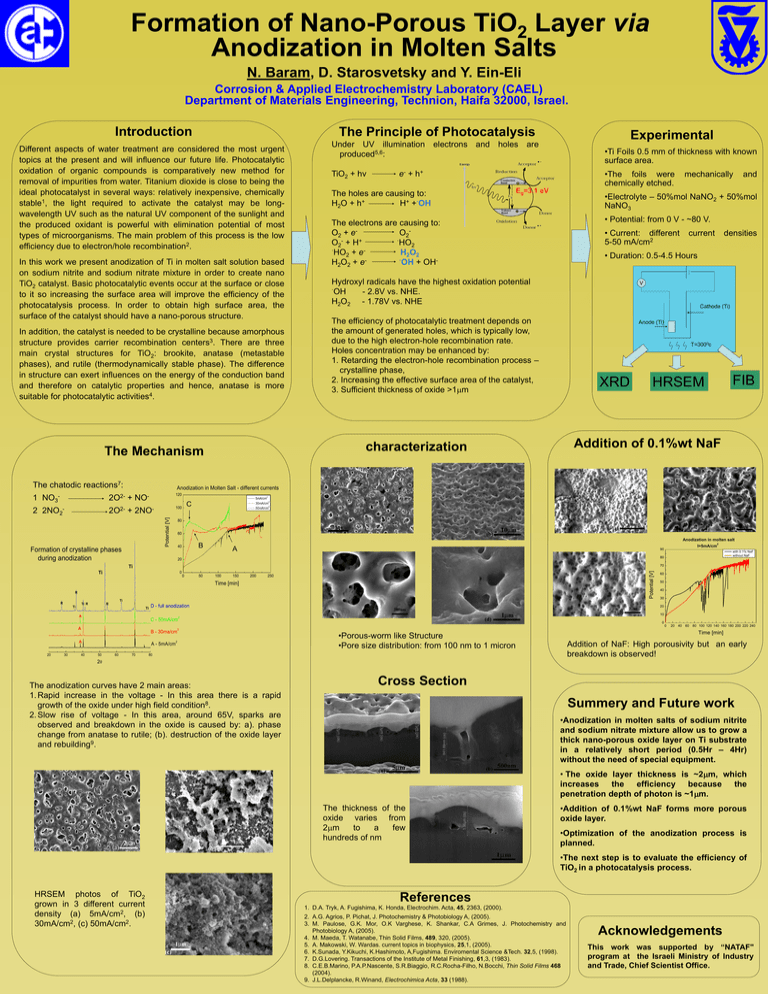
Formation of Nano-Porous TiO2 Layer via Anodization in Molten Salts N. Baram, D. Starosvetsky and Y. Ein-Eli Corrosion & Applied Electrochemistry Laboratory (CAEL) Department of Materials Engineering, Technion, Haifa 32000, Israel. Introduction The Principle of Photocatalysis Different aspects of water treatment are considered the most urgent topics at the present and will influence our future life. Photocatalytic oxidation of organic compounds is comparatively new method for removal of impurities from water. Titanium dioxide is close to being the ideal photocatalyst in several ways: relatively inexpensive, chemically stable1, the light required to activate the catalyst may be longwavelength UV such as the natural UV component of the sunlight and the produced oxidant is powerful with elimination potential of most types of microorganisms. The main problem of this process is the low efficiency due to electron/hole recombination2. In this work we present anodization of Ti in molten salt solution based on sodium nitrite and sodium nitrate mixture in order to create nano TiO2 catalyst. Basic photocatalytic events occur at the surface or close to it so increasing the surface area will improve the efficiency of the photocatalysis process. In order to obtain high surface area, the surface of the catalyst should have a nano-porous structure. In addition, the catalyst is needed to be crystalline because amorphous structure provides carrier recombination centers3. There are three main crystal structures for TiO2: brookite, anatase (metastable phases), and rutile (thermodynamically stable phase). The difference in structure can exert influences on the energy of the conduction band and therefore on catalytic properties and hence, anatase is more suitable for photocatalytic activities4. 2O2- 2 2NO2- TiO2 + hv •Ti Foils 0.5 mm of thickness with known surface area. •The foils were chemically etched. e - + h+ The holes are causing to: H2O + h+ H+ +·OH Eg=3.1 eV + 120 NO- Formation of crystalline phases during anodization •Electrolyte – 50%mol NaNO2 + 50%mol NaNO3 • Potential: from 0 V - ~80 V. The electrons are causing to: O2 + eO2·HO O2- + H+ 2 ·HO + eH2O2 2 ·OH + OHH2O2 + e- • Current: different 5-50 mA/cm2 Hydroxyl radicals have the highest oxidation potential ·OH - 2.8V vs. NHE. H2O2 - 1.78V vs. NHE V The efficiency of photocatalytic treatment depends on the amount of generated holes, which is typically low, due to the high electron-hole recombination rate. Holes concentration may be enhanced by: 1. Retarding the electron-hole recombination process – crystalline phase, 2. Increasing the effective surface area of the catalyst, 3. Sufficient thickness of oxide >1mm Anode (Ti) Cathode (Ti) T=3000c XRD 5mA/cm 2 30mA/cm 2 50mA/cm C 60 Anodization in molten salt B 40 2 A with 0.1% NaF without NaF 80 20 70 0 50 100 150 200 Potential [V] 0 250 Ti R Ti D - full anodization 60 50 40 30 20 10 A C - 50mA/cm 2 0 0 A B - 30ma/cm A 40 I=5mA/cm 90 R 30 FIB HRSEM Addition of 0.1%wt NaF Time [min] 20 densities 80 Ti Ti R current • Duration: 0.5-4.5 Hours Ti Ti and 2 100 2O2- + 2NO- R mechanically Anodization in Molten Salt - different currents Potential [V] 1 NO3 - Under UV illumination electrons and holes are produced5,6: characterization The Mechanism The chatodic reactions7: Experimental A - 5mA/cm 50 60 70 2 2 20 40 60 80 100 120 140 160 180 200 220 240 Time [min] •Porous-worm like Structure •Pore size distribution: from 100 nm to 1 micron Addition of NaF: High porousivity but an early breakdown is observed! 80 2 The anodization curves have 2 main areas: 1. Rapid increase in the voltage - In this area there is a rapid growth of the oxide under high field condition8. 2. Slow rise of voltage - In this area, around 65V, sparks are observed and breakdown in the oxide is caused by: a). phase change from anatase to rutile; (b). destruction of the oxide layer and rebuilding9. Cross Section Summery and Future work •Anodization in molten salts of sodium nitrite and sodium nitrate mixture allow us to grow a thick nano-porous oxide layer on Ti substrate in a relatively short period (0.5Hr – 4Hr) without the need of special equipment. • The oxide layer thickness is ~2mm, which increases the efficiency because the penetration depth of photon is ~1mm. The thickness of the oxide varies from 2mm to a few hundreds of nm •Addition of 0.1%wt NaF forms more porous oxide layer. •Optimization of the anodization process is planned. •The next step is to evaluate the efficiency of TiO2 in a photocatalysis process. HRSEM photos of TiO2 grown in 3 different current density (a) 5mA/cm2, (b) 30mA/cm2, (c) 50mA/cm2. References 1. D.A. Tryk, A. Fugishima, K. Honda, Electrochim. Acta, 45, 2363, (2000). 2. A.G. Agrios, P. Pichat, J. Photochemistry & Photobiology A, (2005). 3. M. Paulose, G.K. Mor, O.K Varghese, K. Shankar, C.A Grimes, J. Photochemistry and Photobiology A, (2005). 4. M. Maeda, T. Watanabe, Thin Solid Films, 489, 320, (2005). 5. A. Makowski, W. Wardas. current topics in biophysics, 25,1, (2005). 6. K.Sunada, Y.Kikuchi, K.Hashimoto, A,Fugishima. Enviromental Science &Tech. 32,5, (1998). 7. D.G.Lovering. Transactions of the Institute of Metal Finishing, 61,3, (1983). 8. C.E.B.Marino, P.A.P.Nascente, S.R.Biaggio, R.C.Rocha-Filho, N.Bocchi, Thin Solid Films 468 (2004). 9. J.L.Delplancke, R.Winand, Electrochimica Acta, 33 (1988). Acknowledgements This work was supported by “NATAF" program at the Israeli Ministry of Industry and Trade, Chief Scientist Office.











