Ozone, UV and nanoparticles
advertisement

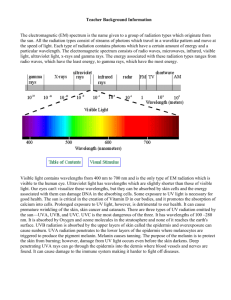
STEM ED/CHM Nanotechnology 2011 Ozone, UV, and Nanoparticles Mort Sternheim STEM Education Institute mort@umassk12.net Today’s agenda 1. Ozone and ultraviolet light 2. Nanoparticles and sunscreen 3. Hands on activity (brief) Sunscreen PowerPoint and activities based on NanoSense web site: http://nanosense.org/activities/clearsunscreen The big ideas • • • • Ultraviolet light causes skin damage and cancer Ozone in the stratosphere blocks UV Sunscreen blocks UV, partly Nanoparticles in sunscreen improve blocking 1. Ozone and Ultraviolet Light What is ozone? • Ordinary oxygen gas: O2 (2 oxygen atoms) • Ozone: O3 (3 oxygen atoms) • Polar molecule, like water • Ozone is much more reactive, unstable • Pale blue, poisonous gas Bad! • Absorbs ultraviolet radiation! Good! The Sun’s radiation spectrum Most of the sun’s radiation is Ultraviolet (UV), Visible & Infrared (IR) : • ~ 43% is in the visible range • ~ 49% is in the near infrared range • ~ 7% is in the ultraviolet range . • < 1% is x-rays, gamma rays, radio waves Source: Adapted from http://www.ucar.edu/learn/imgcat.htm Some types of electromagnetic radiation • The sun emits several kinds of electromagnetic radiation: Visible (Vis), Infrared (IR) and Ultra Violet (UV). Note the split into UVA, UVB, UVC High Energy Low Energy • Each kind is distinguished by a characteristic wavelength, frequency and energy • Higher energy radiation can damage our skin Source: http://www.arpansa.gov.au/is_sunys.htm What is Radiation? • Light radiation is often thought of as a wave with a wavelength (l), speed (c), and frequency (f) related by • Since c (the speed of light) is constant, the wavelength and frequency are inversely related • This means that light with a short wavelength will have a high frequency and visa versa. Source: http://www.pueblo.gsa.gov/cic_text/health/sun_uv/sun-uv-you.htm Radiation energy comes in packets or photons • The size of an energy packet or photon (E) is determined by the frequency of the radiation (f) E • • Radiation with a higher frequency has more energy in each packet The amount of energy in a packet determines how it interacts with our skin Ef f Skin Damage • Very high energy radiation (UVC) is currently blocked by the ozone layer • High energy radiation (UVB) does the most immediate damage (sunburns) • But lower energy radiation (UVA) can penetrate deeper into the skin, leading to long term damage Source: N.A. Shaath. The Chemistry of Sunscreens. In: Lowe NJ, Shaath NA, Pathak MA, editors. Sunscreens, development, evaluation, and regulatory aspects. New York: Marcel Dekker; 1997. p. 263-283. Good ozone • In the stratosphere, absorbs 97+ % of solar UV, protecting life from harm • Produced by solar UV light from O2 : – O2 + UV (radiation < 240 nm) → 2 O – O + O2 → O3 • Ozone – oxygen cycle: – O3 + UV (< 320 nm) → O2 + O • This cycle heats the atmosphere slightly, so ozone is a minor greenhouse gas Ozone is the Earth’s natural sunscreen 100 60 Thermosphere UVc - 100% Absorption Mesosphere UVb - 90% Absorption UVa - 50% Absorption & Scattering 50 40 60 30 40 20 20 10 Troposphere 0 0 2 4 6 Ozone (parts per million) 11 8 0 Altitude (miles) Altitude (km) 80 Ozone layer • Ozone in stratosphere, 10 to 50 km above surface • Ozone Can be depleted by free radical catalysts – NO, OH, Cl, Br – from natural sources • Also from chlorofluorocarbons (CFCs) (freons) and bromofluorocarbons (halons) – UV light produces free Cl, Br radicals – Cl, Br catalyze chain reactions destroying ~100,000 ozone molecules • Used in aerosols, refrigerators, air conditioners, fire extinguishers Chemicals that Destroy Stratospheric Ozone Other gases Methyl chloroform (CH3CCl 3) HCFCs (e.g., HCFC-22 = CHClF 2) CFC-113 (CCl 2FCClF 2) 1% 4% 5% 7% 3400 3000 Other halons 20 Carbon tetrachloride (CCl 4) 12% 14% Halon-1301 (CBrF3) Halon-1211 (CBrCIF 2) 15 CFC-11 (CCl 3F) 20% 5-20% 2000 23% 4% Methyl bromide (CH3Br) 10 CFC-12 (CCl 2F2) 1000 5 32% 0 Natural sources 16% 27-42% Methyl chloride (CH3Cl) 0 Very-s hort live d gas e s (e.g., bromoform = CHBr 3) 15% • Cl is much more abundant than Br • Br is about 50 times more effective at O3 destruction From Ozone FAQ - see http://www.unep.org/ozone/faq.shtml Ozone depletion • Stratospheric ozone levels decreasing ~4% per year since ’70’s • More skin cancer? • Larger seasonal decrease in lower altitudes (troposphere) in polar regions: the ozone hole • CFC’s phased out globally by 1996 (Montreal Protocol, 1987) – will take decades to leave atmosphere • Ozone levels have stabilized • Recovery will take decades 2. Nanoparticles and sunscreen • Nanoparticles: 1 to 100 nm in diameter, or about 10 to 1000 atomic diameters • Number of products using nanomaterials is growing very rapidly – Doubling every year? • Clothing, food and beverages, sporting goods, coatings, cosmetics, personal care • Sunscreens: many use nanomaterials – Some labeled as containing nanoparticles – Some not labeled http://www.masspolicy.org/p df/workshop/rejeski.pdf http://www.nanotechproject.org/inventories/consumer/analysis_draft/ Why Use Sunscreen? Too much unprotected sun exposure leads to: • Premature skin aging (e.g. wrinkles) • Sunburns • Skin cancer Sources: http://www.oasishospital.org/previousnews.html; http://wohba.com/archive/2005_03_01_archive.html Skin Cancer Rates are Rising Fast Probability of getting skin cancer: 1930 : 1 in 5,000 2004 : 1 in 65 2050 : 1 in 10… Skin cancer: • Is ~50% of all cancer cases • Has > 1 million cases diagnosed each year • Causes 1 person to die every hour Causes of the increase: • Decreased ozone protection • Increased time in the sun • Increased use of tanning beds Sources: http://www.msnbc.msn.com/id/8379291/site/newsweek/ ; http://www.skincarephysicians.com/skincancernet/whatis.html; http://www.msu.edu/~aslocum/sun/skincancer.htm Sun Radiation Summary Radiation Type Characteristic Wavelength (l) Energy per Photon % of Total Radiation Reaching Earth Effects on Human Skin Visible to Human Eye? UVC ~200-290 nm (Short-wave UV) Increasing Energy ~0% DNA Damage No High Energy (<1% of all UV) Sunburn DNA Damage Skin Cancer No Tanning Skin Aging DNA Damage Skin Cancer No UVB UVA ~290-320 nm (Mid-range UV) ~320-400 nm (Long-wave UV) Vis ~400-700 nm IR ~700-120,000 nm Increasing Wavelength Medium Energy ~.35% (5% of all UV) ~6.5% Low Energy Lower Energy Lowest Energy (95 % of all UV) ~43 % Yes ~49% No Which Sunscreen Should You Use??? New and Improved Now with Nano-Z Broadband Protection Safe for Children SPF 50 Goes on Clear A Brief History of Sunscreens: The Beginning • First developed for soldiers in WWII (1940s) to block “sunburn causing rays” These were called UVB rays WWII soldier in the sun Shorter wavelengths (more energy) called UVC Longer wavelengths (less energy) called UVA Sources: http://www.bbc.co.uk/wiltshire/content/articles/2005/05/05/peoples_war_feature.shtml http://www.arpansa.gov.au/is_sunys.htm A Brief History of Sunscreens: The SPF Rating • Sunscreens first developed to prevent sunburn – Ingredients were good UVB blockers • SPF (Sunscreen Protection Factor) Number – Measures the strength of UVB protection only – Higher SPF # = more protection from UVB – Doesn’t tell you anything about protection from UVA Sources: http://www.shop.beautysurg.com/ProductImages/skincare/14521.jpg and http://www.shop.beautysurg.com/ProductImages/skincare/14520.jpg A Brief History of Sunscreens: The UVA Problem • UVA rays have no immediate visible effects but cause serious long term damage – Cancer – Skin aging • Sunscreen makers working to find UVA blockers – No official rating of UVA protection yet Source: http://www.cs.wright.edu/~agoshtas/fig8.jpg Twenty different skin cancer lesions How do you know if your sunscreen is a good UVA blocker? Proposed UVA Ratings (2010) • Label will state whether there is UVA protection, but not how much • There is no present standard for UVA • Proposed 1 to 4 star system for UVA protection for “low protection” to “highest protection” • Debate: is this dual system confusing or helpful? • Plan has been scrapped New FDA UVA Ratings (2012) • The phrase “broad spectrum” is meant to indicate protection against UVA • Products labeled “broad spectrum” will have to provide equal protection against UVB and UVA • Bathing suits: 3 tbsp every 2 hours Clothing • Ordinary clothing provides a good sun shield when dry (the tighter the weave, the better) but little or no protection when wet • Special sun-protective clothing is costly but works well wet or dry; it is a wise investment for children who tend to stay in or around water for hours. Know Your Sunscreen: Look at the Ingredients • UV blocking agents suspended in a lotion – “Colloidal suspension” • Lotion has “inactive ingredients” – Don’t block UV light • UV blocking agents are “active ingredients” – Usually have more than one kind present • Two kinds of active ingredients – Organic ingredients and inorganic ingredients Source: Original Image Organic Ingredients: The Basics • Organic = Carbon Atoms – Hydrogen, oxygen & nitrogen atoms are also often involved • Structure – Covalent bonds – Exist as individual molecules • Size – Molecular formula determines size – Typical a few to several dozen Å (<10 nm) Sources: http://www.3dchem.com/molecules.asp?ID=135# and original image Octyl methoxycinnamate (C18H26O3) an organic sunscreen ingredient Organic Ingredients: UV Absorption 1. Electrons capture the energy from UV rays 2. They jump to higher energy levels hf=2.48 eV 3. The energy is released as infrared rays which are harmless (each ray is low in energy) 3hf=2.48 eV Source: Adapted from http://www.3dchem.com/molecules.asp?ID=135#and http://members.aol.com/WSRNet/tut/absorbu.htm Organic Ingredients: Absorption Range • Organic molecules only absorb UV rays whose energy matches difference between electron energy levels – Different kinds of molecules have different peaks and ranges of absorption – Using more than one kind of ingredient (molecule) gives broader protection One Ingredient Two Ingredients Three Ingredients Source: Graphs adapted from http://www.aims.gov.au/pages/research/projects/sunscreens/pages/sunscreens02.html Organic Ingredients: Absorption Range cont. • Most organic ingredients that are currently used were selected because they are good UVB absorbers – The FDA has approved 15 organic ingredients • Sunscreen makers are trying to develop organic ingredients that are good UVA blockers – Avobenzone (also known as Parasol 1789) is a new FDA approved UVA blocker Source: http://jchemed.chem.wisc.edu/JCEWWW/Features/MonthlyMolecules/2004/Oct/JCE2004p1491fig4.gif How are inorganic sunscreen ingredients different from organic ones? How might this affect the way they absorb UV light? Inorganic Ingredients: The Basics • Atoms involved – Zinc or Titanium – Oxygen • Structure – Ionic molecules: ZnO, TiO2 – Cluster of ions – Formula unit doesn’t dictate size • Cluster (particle) size – Varies with # of ions in cluster – ~10 nm – 300 nm Detail of the ions in one cluster Group of TiO2 particles Source: http://www.microspheres-nanospheres.com/Images/Titania/TIO2%20P7.jpg and image adapted from http://www.cse.clrc.ac.uk/msi/projects/ropa.shtml Inorganic Ingredients: Cluster Size • Inorganic ingredients come in different cluster sizes (sometimes called “particles”) – Different number of ions can cluster together – Must be a multiple of the formula unit • ZnO always has equal numbers of Zn and O atoms • TiO2 always has twice as many O as Ti atoms ~100 nm TiO2 particle Source: Images adapted from http://www.cse.clrc.ac.uk/msi/projects/ropa.shtml ~200 nm TiO2 particle Inorganic Ingredients: UV Absorption • Inorganics have a different absorption mechanism than organics • Absorb consistently through whole UV range up to ~380nm Source: Graph adapted from http://www.aims.gov.au/pages/research/projects/sunscreens/pages/sunscreens02.html Why not use inorganics? • Appearance Matters • Traditional inorganic sunscreens have appear white on our skin • Many people don’t like how this looks, so they don’t use sunscreen with inorganic ingredients • Of the people who do use them, most apply too little to get full protection Source: http://www.4girls.gov/body/sunscreen.jpg Why Do They Appear White? • Traditional ZnO and TiO2 clusters are large – (> 200nm) • Large clusters scatter visible light – (400-700 nm) – Maximum scattering occurs for wavelengths twice as large as the clusters • The scattered light is reflected to our eyes, appearing white Source: Original image Organic Sunscreen Molecules are Too Small to Scatter Light ~200 nm TiO2 particle Methoxycinnamate (Inorganic) (Organic) (Note that these images are not drawn to scale) Source: Images adapted from http://www.cse.clrc.ac.uk/msi/projects/ropa.shtml and http://www.3dchem.com/molecules.asp?ID=135# Waves and obstacles • Waves go around small obstacles • Waves scatter all around from obstacles of sizes comparable to a wavelength • Water wave (ripple tank) simulation: http://www.falstad.com/ripple/ What could we do to inorganic clusters to prevent them from scattering visible light? Source: Adapted from http://www.loc.gov/rr/scitech/mysteries/images/sunscreen2.jpg Nanosized Inorganic Clusters • Maximum scattering occurs for wavelengths twice as large as the clusters – Make the clusters smaller (100 nm or less) and they won’t scatter visible light Source: Graph adapted from http://www.aims.gov.au/pages/research/projects/sunscreens/pages/sunscreens02.html In Summary… • Nanoparticle sunscreen ingredients are small inorganic clusters that: – Provide good UV protection by absorbing both UVB and UVA light – Appear clear on our skin because they are too small to scatter visible light Source: http://www.smalltimes.com/images/st_advancednanotech_inside_.jpg Essential Questions: Time for Answers 1. What are the most important factors to consider in choosing a sunscreen? 2. How do you know if a sunscreen has “nano” ingredients? 3. How do “nano” sunscreen ingredients differ from other ingredients currently used in sunscreens? 3. Testing sunscreen activity • Use UV sensitive beads • Compare opacity/ transparency of samples for visible light and UV light • Beads absorb UV from 300 nm to 360 nm (UV A is 320 – 400 nm, UV B is 280 – 320 nm) • Make UV detector necklaces