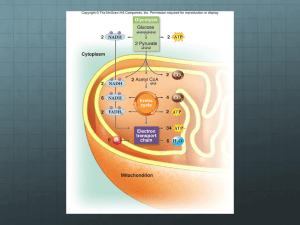
The Respiratory Chain and ATP Synthase
advertisement

Respiratory control: Rate of oxidative phosphorylation is determined by the need for ATP 1. Electron transport and ATP synthesis is coupled. 2. Electrons do not flow through the electron-transport chain to O2 unless ADP is simultaneously phosphorylated to ATP. 3. When [ADP] rises, the rate of oxid. phos. increases to meet the ATP needs. (This is the case in active muscle) 4. The regulation of the rate of oxid. phos. by the ADP level is called Respiratory control or Acceptor control. Respiratory control: [ADP] affects the rate of TCA cycle In a resting muscle, [ADP] is low, NADH and FADH2 are not consumed by the ETC. Less NAD+ and FAD available, thus the TCA cycle slows. As [ADP] rises and oxid phos. speeds up, NADH and FADH2 are oxidized, leading to a more active TCA cycle. UCP-1, Brown Fat and uncoupling Some organisms have the ability to uncouple oxid. Phos. from ATP synthesis to generate heat. This is used to maintain body temp in hibernating animals or newborn animals. In response to a temp drop, the release of hormones leads to the liberation of free fatty acids from triacylglycerols that in turn activate UCP-1. UCP-1 (uncoupling protein 1) forms a pathway for the flow of H+ from the cytoplasm to the matrix. UCP-1 generates heat by short-circuiting the mitochondrial proton battery. The tissue with high UCP-1 called brown fat. (combination of greenish cytochromes and red hemoglobin in blood supply) Site of action of some inhibitors of electron transport Inhibits electron flow in Complex I Prevents the utilization of NADH as a substrate. Inhibits electron flow from Cyt bH Cyanide (CN-), azide (N3- ) react with the ferric (Fe3+ ) form of heme a3. Carbon monoxide (CO): Inhibits the ferrous (Fe2+) form. Inhibition of ATP synthesis Oligomycin and DCCD (dicyclohexylcarbodiimide) Prevent the influx of protons through ATP sythase. In the presence of these drugs, the electron-transport chain ceases to operate. Thus, further confirming that electron transport and ATP synthesis are normally tightly coupled. Uncoupling electron transport form ATP synthesis Cytoplasm The compound 2,4-dinitrophenol (DNP) acts as a proton ionophore, that is, it binds protons on one side of a membrane, and being fat-soluble it drifts to the opposite side where it loses the protons. In the presence of DNP, electron transport from NADH to O2 is normal, but no ATP synthesis, since the proton gradient is disrupted. This leads to further increased O2 consumptioin and NADH oxidation. Eventually, large amounts of metabolic fuels are consumed, but no energy is captured as ATP. Energy is releasd as heat. DNP is used as a weight-loss drug for some people. Soviet soldiers were given DNP to keep warm during the long Russian winters. Inhibition of ATP export ATP-ADP translocase can be inhibited by two drugs: a plant glycoside, atractyloside an antibiotic from a mold, bongkrekic acid Atractyloside binds to the enzyme when its nucleotide site faces the cytoplasm. Bongkrekic acid binds to the enzyme when its nucleotide site faces the matrix. In the presence of these inhibitors, oxid. phos. stops--supporting that the translocase is essential for maintaining adequate amounts of ADP to accept the energy. Coupling Electron Transport and Ox/Phos ATP Synthesis Starting materials Add ADP to stimulate oxid. phos. and O2 consumption, [O2] decreases dramatically [O2] Rotenone and amytal block Complex I function, NADH oxidation is inhibited, no more O2 consumption. Succinate can bypass Rotenone or amytal effects, since electrons enter through QH2. Oxid. phos. resumes---[O2] decreases again. time Until ADP is completed used Coupling Electron Transport and Ox/Phos ATP Synthesis Starting materials Add ADP to stimulate O2 consumption [O2] decreases dramatically Antimycin A inhibits Complex III, oxid. phos stops. Succinate addition can not rescue, because antimycin A acts downstream of QH2. [O2] Ascorbate addition can rescue, because it can give up electrons to cytochrome C (Ascorbate reduces cyto c) Until ADP is used time Coupling Electron Transport and Ox/Phos ATP Synthesis Cyanide inhibits complex IV Succinate can not rescue, because cyanide acts downstream of QH2. Ascorbate can not rescue, because cyanide acts downstream of cytochrome c. [O2] DNP can not rescue, because it destroys H+ gradient. time Coupling Electron Transport and Ox/Phos ATP Synthesis Starting materials Add ADP to stimulate O2 consumption [O2] decreases dramatically [O2] Oligomycin inhibits ATP synthase by preventing influx of protons through ATP synthase. Since electron transport is coupled to ATP synthesis, oxid. phos. stops. DNP can transport protons back to matrix from cytoplasm---loss of respiratory control leads to increased O2 consumption. time Electron transport chain inhibitors and substrates Ascorbate + TMPD Antimycin A Complex I Complex II Rotenone Amytal Complex III Sodium azide Cyanide, CO Complex IV • • • Tetramethylphenylenediamine (TMPD) Ascorbic acid maintains TMPD (an artifical electron donor) in a reduced state. Reduced TMPD donates electrons to cytochrome c. Summary Glycerol 3-P shuttle Fig. 16-9 Comparison of the 2 DIFFERENT Ways to Make ATP Location Requires membrane integrity, electron transport and ATP synthase Role of substrate High energy intermediate formed High energy bonds formed/molecule substrate Contribution to human ATP consumption Nucleotide Synthesized Oxidative Phosphorylation Substrate Level Phosphorylation MTCH membrane Cytoplasm MTCH matrix Yes No Source of e- Direct participant No (proton gradient) Yes >1 1 2.5 for NADH, 1.5 for FADH2 > 90% <10% ATP ATP, GTP Mitochondrial diseases LHON: Leber hereditary optic neuropathy A form of blindness as a result of mutations in Complex I Some of these mutations impair NADH utilization, whereas other block electron transfer to Q. Mutations in Complex I are the most frequent cause of mitochondrial diseases. Organs that are highly dependent on oxid. phos., such as the nervous system and the heart, are most vulnerable to mutations that cause mitochrondrial diseases. Oxygen metabolism and toxcity Dual role for molecular O2 Ideal electron terminator in Oxid.Phos. High affinity for electrons provides a large thermodynamic driving force Partial reduction of O2 results in potential hazardous compounds ROS – Reactive Oxygen Species Destroys biologically important compounds Proteins, lipids and DNA - Oxidation Most common radicals formed Fe 2+ DNA & Lipids O2 accept one electron at a time Potential to form free radicals 1-4% of O2 at end of Mitochondrial ETC become free radicals when young. Increases with age Molecular Defenses - Antioxidants Sequestration of transition metals: eg. Fe2+ as ferritin (protein that bind Iron) Superoxide dismutase (SOD): 2•O2 + 2H+ ====> H2O2 + O2 Catalase 2H2O2 ====> 2H2O + O2 Glutathione peroxidase: H2O2 + 2GSH ====> 2H2O + GS-SG Other Radical Scavengers: Vit. C (water soluble) – ascorbate (reducing agent) Vit. E. (Lipid soluble) Prevents lipid oxidation Reactive oxygen species and antioxidants that can reduce them Reactive species Antioxidant Superoxide radical O2- superoxide dismutase, vitamin C Hydrogen peroxide H2O2 catalase, glutathione peroxidase Peroxyl radical ROO vitamin C, vitamin E Lipid peroxyl radical LOO vitamin E Hydroxyl radical OH vitamin C Problems Potent poisons. What is the effect of each of the following inhibitors on e transport and ATP formation by the respiratorty chain? a) Azide: It blocks e transport and proton pumping at Complex IV. b) Atractyloside: It block e transport and ATP synthesis by inhibiting the exchange of ATP and ADP across the inner mitochondrial membrane. c) Rotenone: It blocks e transport and proton pumping at Complex I. d) DNP: It blocks ATP synthesis without inhibiting e transport by dissipating the proton gradient. e) CO: It blocks e transport and proton pumping at Complex IV. f) Antimycin A: It blocks e transport and proton pumping at Complex III. Problems-continued A question of coupling. What is the mechanistic basis for the observation that the inhibitors of ATPase also lead to an inhibition of the e-transport chain? A: If the proton gradient is not dissipated by the influx of protons into a mitochondrion with the generation of ATP, eventually the outside of the mitochondrion develops such a large positive charge that the electrontransport chain can no longer pump protons against the gradient. Cyanide antidote. The immediate administration of nitrite is a highly effective treatment for cyanide poisoning. What is the basis for the treatment? A: --Cyanide is lethal since it binds to ferric (Fe3+) form of cytochrome oxidase thus inhibits oxid. phos. --Nitrite oxidizes ferrohemoglobin (Fe2+) to ferrihemoglobin (Fe3+), which also binds to cyanide. ---Thus, ferrihemoglobin competes with cytochrome oxidase for cyanide. ---The competition is therapeutically effective because the amount of ferrihemoglobin that can be formed without impairing O2 transport is much greater than the amount of cytochrome oxidase. Problems-continued Identifying the inhibition. How do you design an experiment to determine whether a chemical is an e-transport-chain inhibitor or an inhibitor of ATP synthase? A: ----Add the inhibitor with and without an uncoupler, and monitor the rate of O2 consumption. ----If the O2 consumption increases again in the presence of inhibitor and uncoupler, the inhibitor must be inhibiting ATP synthase. ---If the O2 consumption is not affected in the presence of inhibitor and uncoupler, the inhibitor is inhibiting the e-transport chain. ---Remember that DNP can rescue the effect of oligomycin, an inhibitor of ATP synthase, but can not rescue the effect of cyanide, an inhibitor of Complex IV. Problems-continued Reduced Oxidized Reduced Oxidized Reduced --The sequence of e carriers can be determined by the effects of inhibitors of e transfer on the oxid. state of each carrier. --In the presence of e donor and O2, Each inhibitor causes a characteristic Pattern of oxidized/reduced carriers. --Those before the block become reduced (blue) and those after the Block become oxidized (red). Degree of reduction of e carriers in the respiratory chain. The Degree of reduction of e carriers in the respiratory chain is determined by the conditions existing in the mitochond. For example, when the supply of NADH and O2 is abundant, the steady-state degree of reduction of the carriers decreases as e pass from the substrate to O2. When e transfer is blocked, the carriers before the block become more reduced while those beyond the block become more oxidized. For each of the conditions below, predict the state of oxidation of each carrier in the respiratory chain (ubiquinone and cytochromes b, c1, c and a+a3). Problems-continued a) Abundant supply of NADH and O2 A: Early carriers more reduced; later carriers more oxidized. b) Abundant supply of NADH but O2 exhausted A: All carries reduced; in the absence of O2, the reduced carries are not re-oxidized. c) Abundant supply of O2 but NADH exhausted A: All carriers oxidized. d) Abundant supply of NADH and O2 but rotenone added A: NADH is reduced, all others are oxidized. e) Abundant supply of NADH and O2 but antimycin A added A: NADH, ubiquinone and cytochrome b are reduced. Cyto c1, cyt c, cyt (a +a3) are oxidized. f) Abundant supply of NADH and O2 but cyanide added A: All carriers reduced. CN- blocks the reduction of O2 catalyzed by cytochrome oxidase. Problems-continued The effect of Rotenone and Antimycin A on e transfer. Rotenone, a toxic nature product from plants, strongly inhibits NADH dehydrogenase of insect and fish mitochondria. Antimycin A, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. a) Explain why rotenone ingestion is lethal to some insect and fish species A: The inhibition of NADH dehydrogenase by rotenone decreases the rate of e flow through the respiratory chain, which in turn decreases the rate of ATP synthesis. If this reduced rate is unable to meet the organism’s ATP requirements, the organism dies. b) Explain why antimycin A is a poison A: Antimycin A strongly inhibits the oxidation of UQ in the respiratory chain, reducing the rate of e transfer and leading to the consequences described in a. c) Assuming that rotenone and antimycin A are equally effective in blocking their respective sites in the e transfer chain, which would be a more potent poison? Explain. A: Because antimycin A blocks all e flow to oxygen, it is a more potent poison than rotenone, which blocks e flow from NADH but not from FADH2. Problems-continued Uncouplers of Oxidative Phosphorylation. In normal mitochondria the rate of e transfer is tightly coupled to the demand for ATP. Thus when ATP is demanded at a high rate, e transfer is rapid. Under such conditions of tight coupling, the no of ATP produced per atom of oxygen consumed when NADH is the e donor---know as the P/O ratio---is close to 3. a) Predict the effect of a relatively low concentration of an uncoupling agent on the rate of e transfer and the P/O ratio. A: The rate of e transfer necessary to meet the ATP demand increases, and thus the P/O ratio decreases. b) The ingestion of uncouplers (high concentration) causes profuse sweating and an increase in body temperature. Explain this phenomenon in molecular terms. What happened to P/O ratio under this condition? A: High concentrations of uncoupler produce P/O ratios near zero. The P/O ratio decreases, and more fuel must therefore be oxidized to generate the same amount of ATP. The extra heat released by this oxidation raises the body temperature. c) The uncoupler 2, 4-dinitrophenol was once prescribed as a weight-reducing drug. How can this agent sever as a reducing aid? DNP is no longer prescribed because some deaths occurred following its use. Why? A: Increased activity of the respiratory chain in the presence of DNP requires the degradation of additional fuel. By oxidizing more fuel (including fat reserves) to produce the same amount of ATP, the body loses weight. When the P/O ratio approaches zero, the lack of ATP results in death. Problems-continued Mode of action of DCCD. When DCCD (dicyclohexylcarbodiimide) is added to a suspension of tightly coupled, actively respiring mitochondria, the rate of e transfer (measured by O2 consumption) and the rate of ATP production dramatically decrease. If a solution of 2,4-dinitrophenol (DNP) is now added to the inhibited mitochondrial preparation. O2 consumption returns to normal but ATP production remains inhibited. a) What process in e transfer or oxidative phosphorylation is affected by DCCD? A: The formation of ATP is inhibited. b) Why does DCCD affect the O2 consumption of mitochondria? Explain the effect of DNP on the inhibited mitochondrial preparation. A: The formation of ATP is tightly coupled to e transfer. DNP is an uncoupler of oxidative phosphorylation. c) Which of the following inhibitors does DCCD most resemble in its action: antimycin A, rotenone, or oligomycin? A: Oligomycin. Problems-continued Mode of Demerol. When the widely prescribed painkiller Demerol is added to a suspension of respiring mitochondria, the ratios NADH/NAD+ and Q/QH2 increase. Which e-transport complex is inhibited by Demerol? A: Demerol interacts with Complex I and prevents e transfer from NADH to Q. The concentration of NADH increases since it cannot be reoxidized to NAD+. The concentration of Q increases since e from QH2 are transferred to O2 but Q is not reduced back to QH2. When the antibiotic myxothiazole is added to respiring mitochondria, the ratio cytochrome c1 (Fe3+) / cytochrome c1 (Fe2+) increases. Where does the antibiotic inhibit the e-transport chain? A: The drug inhibits e transfer from QH2 to cytochrome c1 in Complex III. The oxidized form of cytochrome c1 predominates since Fe3+ cannot be reduced by e from QH2. Problems-continued Mode of atractyloside. Atractyloside is a toxic glycoside from a Mediterranean plant that specifically inhibits the ADP/ATP carrier. Why does the drug cause e transport to be inhibited as well? A: ---Oxid. phos. Is normally tightly coupled to e transport. ---Unless ADP can continue to be translocated into the mitochondria matrix for the ATP synthase reaction, oxidative phosphorylation is not possible and respiration will cease. (i.e., e transport will stop and no oxygen will be reduced to water). Effects of inhibitors. The O2-consumption curve of a dilute, well-buffered suspension of mitochondria containing an excess of ADP and Pi takes the form as below. Sketch the curves obtained under the following conditions. Problems-continued a) Amytal is added at time t=1 A: O2 consumption ceases because amytal blocks e transport in Complex I. b) Amytal is added at t=1 and succinate is added at t=2 A: Electrons from succinate bypass the amytal block by entering the e-transport chain at Complex II and thereby restore e transport through Complexes III and IV. Rescue. c) CN- is added at t=1 and succinate is added at t=2 A: CN- blocks e transport in Complex IV, after the point of entry of succinate. No rescue. d) Oligomycin is added at t=1 and DNP is added at t=2 A: Oligomycin blocks oxid. phos. and hence O2 consumption. DNP uncouples e transport from oxid. phos, and thereby permits O2 consumption to resume. Rescue.