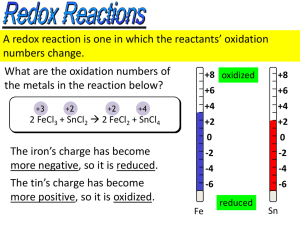
Redox Reactions
advertisement

Higher Chemistry Unit 3 Section 5 Redox Reactions Multiple Choice Questions This is designed to be used by teachers to help students develop skills in answering multiple choice questions. Douglas Racey, Waid Academy, Anstruther, Fife, Scotland Higher Chemistry Unit 3 - Section 5 1. Redox Reactions Multiple Choice Questions If 96,500C of electricity are passed through separate solutions of copper(II)chloride and nickel(II) chloride, then A. B. C. D. equal masses of copper and nickel will be deposited the same number of atoms of each metal will be deposited the metals will be plated on the positive electrode different numbers of moles of each metal will be deposited Answer B. Higher Chemistry Unit 3 - Section 5 2. Redox Reactions Multiple Choice Questions The ion-electron equations for a redox reaction are 2I-(aq) I2(aq) + 2eMnO4-(aq) + 8H+(aq) + 5e- Mn2+(aq) + 4H2O(l) How many moles of iodide ions are oxidised by one mole of permanganate ions? A. B. C. D. 0.2 0.4 2 5 Answer D. Higher Chemistry Unit 3 - Section 5 3. Redox Reactions Multiple Choice Questions In which of the following reactions is hydrogen gas acting as an oxidising agent? A. B. C. D. H 2 + C 2H 4 H2 + Cl2 H2 + 2Na H2 + CuO Answer C 2H 6 2HCl 2NaH H2O + Cu D. Higher Chemistry Unit 3 - Section 5 4. Redox Reactions Multiple Choice Questions Which of the following is a redox reaction? A. B. C. D. NaOH + HCl NaCl + H2O Zn + 2HCl ZnCl2 + H2 NiO + 2HCl NiCl2 + H2O CuCO3 + 2HCl CuCl2 + H2O + CO2 Answer B. Higher Chemistry Unit 3 - Section 5 5. Redox Reactions Multiple Choice Questions The reduction of copper ions during electroplating can be represented as: Cu2+(aq) + 2e Cu(s) What is the quantity of electricity needed to produce 0.25 moles of copper? A. B. C. D. 24 125 C 48 250 C 96 500 C 193 000 C Answer B. Higher Chemistry Unit 3 - Section 5 6. Redox Reactions Multiple Choice Questions Which of the following is a redox reaction? A. B. C. D. Mg + 2HCl MgCl2 + H2 MgCO3 + 2HCl MgCl2 + H2O + CO2 MgO + 2HCl MgCl2 + H2O Mg(OH) 2+ 2HCl MgCl2 + H2O Answer A. Higher Chemistry Unit 3 - Section 5 7. Redox Reactions Multiple Choice Questions The iodate ion, IO3-, can be converted to iodine. Which is the correct ion-electron equation for the reaction? A. B. C. D. 2IO3- + 12H+ +12e- 2I- + 6H2O IO3- + 6H+ +7e- I- + 3H2O 2IO3- + 12H+ +11e- I2 + 6H2O 2IO3- + 12H+ +10e- I2 + 6H2O Answer D. Higher Chemistry Unit 3 - Section 5 Redox Reactions Multiple Choice Questions 8. HgCl2(aq) + SnCl2 (aq) Hg(l) + SnCl4(aq) What ion is oxidised in the above redox reaction? A. B. C. D. Sn2+(aq) Sn4+(aq) Hg2+(aq) Cl-(aq) Answer A. Higher Chemistry Unit 3 - Section 5 Redox Reactions Multiple Choice Questions 9. HgCl2(aq) + SnCl2 (aq) Hg(l) + SnCl4(aq) What ion is the oxidising agent in the above redox reaction? A. B. C. D. Sn2+(aq) Sn4+(aq) Hg2+(aq) Cl-(aq) Answer C. Higher Chemistry Unit 3 - Section 5 Redox Reactions Multiple Choice Questions 10. The ion-electron equations for a redox reaction are 2I-(aq) I2(aq) + 2eMnO4-(aq) + 8H+(aq) + 5e- Mn2+(aq) + 4H2O(l) How many moles of iodide ions are oxidised by one mole of permanganate ions? A. B. C. D. 0.2 0.4 2 5 Answer D.