Tropospheric ozone and OH - Atmospheric Chemistry Modeling Group
advertisement

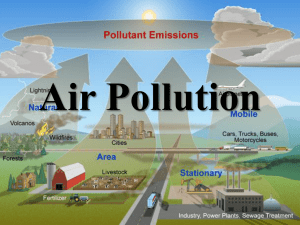
TROPOSPHERIC OZONE AND OXIDANT CHEMISTRY The many faces of atmospheric ozone: In stratosphere: UV shield Stratosphere: 90% of total In middle/upper troposphere: greenhouse gas Troposphere In lower/middle troposphere: precursor of OH, main atmospheric oxidant In surface air: toxic to humans and vegetation TERRESTRIAL RADIATION SPECTRUM FROM SPACE: composite of blackbody radiation spectra emitted from different altitudes at different temperatures THE ATMOSPHERE: OXIDIZING MEDIUM IN GLOBAL BIOGEOCHEMICAL CYCLES Atmospheric oxidation is critical for removal of many pollutants, e.g. • methane (major greenhouse gas) • Toxic gases such as CO, benzene, mercury… • Gases affecting the stratosphere Oxidation Reduced gas EARTH SURFACE Oxidized gas/ aerosol Uptake Emission Reduction Example: Biogeochemical cycle of mercury ANTHROPOGENIC PERTURBATION: fuel combustion mining VOLATILE Hg(0) oxidation (months) WATER-SOLUBLE Hg(II) volcanoes erosion ATMOSPHERE OCEAN/SOIL Hg(0) Hg(II) reduction uplift particulate biological uptake Hg burial SEDIMENTS CO and methane account for most of reduced gas flux to atmosphere • CO observed from space: 50-200 ppb • Methane observed from space: 1650-1800 ppb THE TROPOSPHERE WAS VIEWED AS CHEMICALLY INERT UNTIL 1970 • “The chemistry of the troposphere is mainly that of of a large number of atmospheric constituents and of their reactions with molecular oxygen…Methane and CO are chemically quite inert in the troposphere” [Cadle and Allen, Atmospheric Photochemistry, Science, 1970] • Lifetime of CO estimated at 2.7 years (removal by soil) leads to concern about global CO pollution from increasing car emissions [Robbins and Robbins, Sources, Abundance, and Fate of Gaseous Atmospheric Pollutants, SRI report, 1967] FIRST BREAKTHROUGH: • Measurements of cosmogenic 14CO place a constraint of ~ 0.1 yr on the tropospheric lifetime of CO [Weinstock, Science, 1969] SECOND BREAKTHROUGH: • Tropospheric OH ~1x106 cm-3 predicted from O(1D)+H2O, results in tropospheric lifetimes of ~0.1 yr for CO and ~2 yr for CH4 [Levy, J. Geophys. Res. 1973] THIRD BREAKTHROUGH: • Methylchlroform observations provide indirect evidence for OH at levels of 2-5x105 cm-3 [Singh, Geophys. Res. Lett. 1977] …but direct measurements of tropospheric OH had to wait until the 1990s WHY WAS TROPOSPHERIC OH SO DIFFICULT TO FIGURE OUT? Production of O(1D) in troposphere takes place in narrow band [290-320 nm] solar flux I ozone absorption cross-section s fsI O(1D) quantum yield f MEAN VERTICAL DISTRIBUTION OF ATMOSPHERIC OZONE: only 10% is in the troposphere OZONE CHEMISTRY IN STRATOSPHERE O2 h O O O O2 M O3 M O3 h O2 O (1 D ) O(1 D ) M O M XO O X O2 By contrast, in troposphere: • no photons < 240 nm no oxygen photolysis; • neglible O atom conc. no XO + O loss O2+hv O3+hv UNTIL ~1990, PREVAILING VIEW WAS THAT TROPOSPHERIC OZONE ORIGINATED MAINLY FROM STRATOSPHERE…but that cannot work. • • • Estimate ozone flux FO3 across tropopause (strat-trop exchange) – Total O3 col = 5x1013 moles FO3 = 3x1013 moles yr-1 – 10% of that is in troposphere – Res. time of air in strat = 1.4 yr Estimate CH4 source SCH4: – Mean concentration = 1.7 ppmv SCH4 = 3x1013 moles yr-1 – Lifetime = 9 years Estimate CO source SCO: – Mean concentration = 100 ppbv SCO = 9.7x1013moles yr-1 – Lifetime = 2 months SCO+ SCH4 > 2FO3 e OH would be titrated! We need a much larger source of tropospheric ozone CONSTRAINT ON CROSS-TROPOPAUSE OZONE FLUX FROM OBSERVED OZONE-NOy CORRELATION IN LOWER STRATOSPHERE NOy / N2O 0.073 ( 14%) N2O O(1 D) 2NO NOy chemical family Oxidation products (HNO3, etc.) FN2O = EN2O in lower strat.: NOy / O3 0.0033 ( 12%) FO3 EN2O = 13 Tg N yr-1 (±17%) FN 2O (NOy / N2O) NOy / O3 tropopause 540 140 Tg O3 yr -1 OZONE LOSS IN TROPOSPHERE FO3 540 140 Tg O3 yr tropopause -1 DO3 1000 200 Tg O3 yr -1 deposition Chemical loss: O(1 D) H 2O 2OH OH O3 HO2 O2 HO2 O3 OH 2O2 LO3 4600 700 Tg O3 yr -1 Ozone chemical loss is driven by photolysis frequency J(O3 O(1D)) at 300-320 nm: 0 Closing the tropospheric ozone budget requires a tropospheric chemical source >> FO3 dJ/d, 10-6 s-1 nm-1 J q( )s ( ) I ( )d OZONE PRODUCTION IN TROPOSPHERE Photochemical oxidation of CO and volatile organic compounds (VOCs) catalyzed by hydrogen oxide radicals (HOx) and nitrogen oxide radicals (NOx) HOx = H + OH + HO2 + RO + RO2 NOx = NO + NO2 Oxidation of VOC: Oxidation of CO: RH OH R H 2O CO OH CO2 H H O2 M HO2 M R O2 M RO2 M HO2 NO OH NO2 RO2 NO RO NO2 NO2 h NO O O2 NO2 h NO O3 O O2 M O3 M Net: CO 2O2 CO2 O3 OH can also add to double bonds of unsaturated VOCs RO can also decompose or isomerize; range of carbonyl products RO O2 R ' CHO HO2 HO2 NO OH NO2 Net: RH 4O2 R ' CHO 2O3 H 2O Carbonyl products can react with OH to produce additional ozone, or photolyze to generate more HOx radicals (branching reaction) GLOBAL BUDGET OF TROPOSPHERIC OZONE (Tg O3 yr-1) IPCC (2007) average of 12 models O2 h Chem prod in troposphere 4700 ±700 Chem loss in troposphere 4200 ±500 Transport from stratosphere 500 ±100 Deposition 1000 ±200 O3 Ozone lifetime: 24 ± 4 days STRATOSPHERE 8-18 km TROPOSPHERE h O3 Deposition NO2 NO OH HO2 h, H2O CO, VOC H2O2 OZONE CONCENTRATIONS vs. NOx AND VOC EMISSIONS Box model calculation NOx-limited regime Ridge NOxsaturated regime