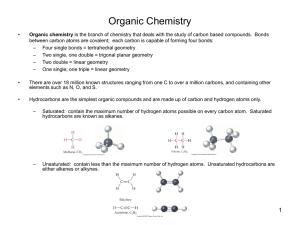
Chemistry 125: Lecture 51
February 14, 2011
This
Cycloaddition
Epoxides
Ozonolysis & Acetals
CH3Li + O=CH2 Analogy
OsO4
For copyright
notice see final
page of this file
Other “Simultaneous” Reagents
Cl2C: (Carbene)
R2BH (Hydroboration)
CH2I2 Zn/Cu (Carbenoid)
O
RC
(Epoxidation)
OOH
O3 (Ozonolysis)
H-metal (Catalytic Hydrogenation)
R-metal (Metathesis, Polymerization)
Wouldn’t it have
(s & p are
been simpler
defined with
to abbreviate
p
respect
to the
arrows
as in
plane
of the
peroxyacid
textbooks?
nuclei)
H
p
All happen
O
together with
s minimal atomic H
displacement
(but not strictly in parallel)
s
s
s
O C
p
H2C
CH2
O
H2C
CH2
Org Syn Prep
(click)
Cl
- K+
O
H2C CH2
- K+
Cl
HO
HO-- Catalysis
O ring strain
H2C CH2
or
H++ Catalysis
O
H2C CH2
H2O
HO
H2C CH2
OH
ethylene glycol
solvents,
20,000,000 tons (antifreeze,
polymers)
billion
-$20
ofHO
which 2/3
O
per year – 3H to
O
polyethers
>20,000 units
2
H2C CH2 - HOH2C CH2
(solvents)
OH
OH
good leaving group
H
O+
H2C CH2
H2O
-
H+
HO
H2C CH2
OH
e.g. J&F Sec. 10.4c pp. 427-430
Regiospecificity
DDEa = 0.12 kcal/mole
55 / 45 = 100.09
Protonated
Isobutylene Oxide
1.61Å
+79
1.47Å
+195
worst
best place
place
for
for Nu
H+-
+141.5
+140.2
Cuprates (Carbon Nucleophiles)
Stereospecificity
More Impressive
Regiospecificity
J&F sec 10.5c 430
Ozonolysis by
Cycloadditions
e.g. J&F sec 10.5a 436-439
Concerted
Transition State
(calc by quantum mech)
_
O
H2C
+
O
O
CH2
Motion along Reaction Coordinate
through Transition State
O3
C2H4
side view
end view
Transition State
Orbital Mixing
makes two new bonds
O3
HOMO
LUMO
HOMO
LUMO
HOMO-1
HOMO
C2H4
Cycloaddition of
Allylic 1,3-Dipoles to Alkenes
7
+
O
O
+
O
O
O
+
O
O
O
O
open structure of O3 (Cf. Lecture 3)
O
O
+
O
Ozone (O3) from the “top”
(rotate back at the top to view the 3 p MOs made from
the 3 “allylic” out-of-plane 2p orbitals of the 3 O atoms)
3 Two ABNs - highest energy p MO.
(I’m not sure why the middle AO looks about the
same size as the terminal ones, it must be larger in
order for 3 to be orthogonal to 1.)
2 One ABN node. Middle AO is absent.
No significant overlap, thus ~ same energy
as isolated 2p AO.
1 No ABN (anti-bonding node)
Middle AO is largest (it overlaps twice)
Another allylic system
CH2-BH-CH2 from “top”
(rotate back to view 3 p MOs)
3
Most of the lower-energy
C AOs were used up
in 1 and 2.
2
Note how C AOs look
larger than O AOs of O3,
because C AO is less dense
near the nucleus)
1 (middle B AO about same
size as C AOs; overlaps twice,
but has lower nuclear charge)
H2C=O O
+
“carbonyl oxide”
p*
p* - p
3
C=O
3
BIG C AO
for this highenergy MO
O
less mixing
pC
p-
pO
2 pO
pO
more mixing
pC=O
node no
longer in
exact center
(better E-match)
1
2
p+p
O
Central O overlaps C better than O, so view as
right O interacting weakly with C=O orbitals.
1
Partly C AO just
looks big (but
also C=O is short,
which makes
CO overlap
important)
Number
of p
electrons
H
B
4
O
2
HC
H
C
O
O
H
H
Makes two bonds Can’t make two bonds
simultaneously for
cycloaddition to alkene!
LUMO
(ends* match p
alkene HOMO)
..
HOMO
(ends match p*
alkene LUMO)
..
* Don’t worry about apparent bad
overlap with the blue lobe of the
central oxygen. It is far enough
away because of the bend in O3.
HC
H
+
O
4
O
Makes two bonds
LUMO
(ends match p
alkene HOMO)
..
LUMO
(No alkene
HOMO match)
HOMO
(No alkene
LUMO match)
..
HOMO
(ends match p*
alkene LUMO)
..
Ozonolysis
+
O O
O
H2C
CH2
e.g. J&F Section 10.5a, pp. 436-439
Ozonolysis
:O
H2C
O
“Molozonide” is rather
unstable because of
HOMO-HOMO mix
in -O-O-O- group.
O
CH2
Undergoes a
“reverse” of the
previous process.
Ozonolysis
to give
carbonyl oxide
and C=O
Re-adds after rotation
(avoids -O-O-O-)
+
O O
H2C
O 2
CH
O
CH2
Undergoes a
“reverse” of the
previous process.
Ozonolysis
O-O
CH2
H2C
O
Ozonide
a Double Acetal
:
H+
HOH
Mechanism for Acid-Catalyzed Hydrolysis of Acetal
SN1
(e.g. J&F pp. 785-787)
First remove RO, and replace it by HO. Process?
+
RO
RO
CH2
H
ROH
+
RO-CH2
CH2
RO
cation unusually stable,
thus easily formed
RO
Now remove second RO, then H (from HO)
ROH
+ H
RO
RO
+
CH2
H-O-CH2
CH2
HO
HO
Process?
E1
O=CH2
(hemiacetal)
Overall Transformation:
H2O + Acetal
H+
+
RO=CH2
Carbonyl + 2 ROH
RO
+
HO
RO
ROH
O=CH
CH2
RO
ROH
CH2
H
H
O
H
Ozonolysis
and hydrogen peroxide
which oxidizes aldehydes to carboxylic acids!
HO-OH
HO-O
O CH2
H2C=O
C O O=CH
HHO
O HH
Ozonide is a Double Acetal
So Double Hydrolysis
Gives Two Carbonyl Compounds
e.g. J&F Sec. 10.5b pp. 440-441
Ozonolysis
Add a reducing agent like (CH3)2S (or Zn) to destroy HOOH and save RCH=O.
Or go with the flow and add more HOOH to obtain a good yield of RCOOH.
What Happens to HOOH + RCHO?
-O
OH
Hydride Shift
H
H
C
O
OH
H
O
R
O
3-membered ring C
with O-O bond is
R
even worse.
R
O
OH
O
C
R
OH
OH- is a bad leaving group from C,
but O-O bond is very weak.
Cf.
B
R
-
-
R
Problem:
Try drawing an analogous acid-catalyzed
mechanism in which HOOH attacks the protonated carbonyl,
then H+ is lost from one O of the HOOH fragment in the
product and added to the other before rearrangement.
-OH
O
-
O
HOH
R C
O
“Nucleophilic”
Addition
to C=O
The nucleophilic addition of methyl
lithium to carbonyl groups* is
formally quite different from these
additions of electrophiles
to alkenes, but the following
transition state analysis reveals
a marked mechanistic similarity.
* which will be discussed in more detail later.
Transition State
Motion
Li-CH3
Li CH3
O CH2
O=CH2
Transition State
Orbital Mixing
Li-CH3
LUMO+2
HOMO
p*
p HOMO
LUMO
O=CH2
Orbital Variety
from Metals
e.g. J&F Sec. 10.5c p. 443
OsO4 and Permanganate
Os or Mn-
HOMO
LUMO
overlaps with alkene p*
e.g. J&F Sec. 10.5c p. 443
OsO4 and Permanganate
H-O-H
O
O
Os
O
H
H 3C
C
O
Os
O
C
O
CH3
H
Os analogue
HO O OH
of
cyclic acetal
H C C CH3
H 3C
H
Osmate Ester
Abigail Batchelder
End of Lecture 51
February 14, 2011
Copyright © J. M. McBride 2011. Some rights reserved. Except for cited third-party materials, and those used by visiting
speakers, all content is licensed under a Creative Commons License (Attribution-NonCommercial-ShareAlike 3.0).
Use of this content constitutes your acceptance of the noted license and the terms and conditions of use.
Materials from Wikimedia Commons are denoted by the symbol
.
Third party materials may be subject to additional intellectual property notices, information, or restrictions.
The following attribution may be used when reusing material that is not identified as third-party content:
J. M. McBride, Chem 125. License: Creative Commons BY-NC-SA 3.0