Chapter 1
Introduction: Themes in the Study of
Life
What are Themes?
• General principles or ideas that occur
over and over.
• In the new AP curriculum, the themes
are “the Big Ideas”.
The 4 Big Ideas: E2 – I2
1. Evolution
2. Energy
3. Information
4. Interactions
The Big Ideas – E2 - I2
1. Evolution – the process of evolution drives the
diversity and unity of life.
2. Energy – biological systems utilize free energy and
molecular building blocks to grow, to reproduce,
and to maintain dynamic homeostasis.
3. Information – living systems store, retrieve,
transmit and respond to information essential to
life processes.
4. Interactions – biological systems interact and these
systems and their interactions possess complex
properties.
Why Big Ideas?
• We will see the Big Ideas at various
times throughout the course.
• The Big Ideas will be the “connectors”
between the content of the course.
Scientific Method Steps
1. Identify the problem.
2. What is already known?
3. Formulate a hypothesis.
4. Conduct an experiment changing one
variable at a time. (Why?)
5. Collect data. Have replicates. (Why?)
6.Compare data to hypothesis.
Does the
data support the hypothesis?
7. Conclusions and new hypothesis
Comment
• Nothing is ever proven in science.
– Can only be disproven
• Experiments either support or fail to
support a particular hypothesis.
• Disproving a hypothesis is as important
as supporting it.
• We will learn about the 7 Science
Practices in Lab.
Chapter Summary
• Big Ideas can provide a common
framework for learning Biology.
• What are the characteristics of Life?
• How does Science work?
• Evolution’s role in the study of Biology.
QUESTIONS FROM CHAPTER 2 and/or 3?
I am assuming you remember and know the basic chemistry information from this chapter. If you
need more information or practice with this information, let me know.
Questions I have for you:
1. Why would weak bonds between molecules be important in the
biological world?
a. brief contact is important for chemical signaling in the brain
b. Hydrogen bonding in DNA
c. Van der Waals Interactions (may occur in proteins)
d. Lock and key fit of biological molecules aids in the
formation of weak bonds.
e. DNA replication
f. Gecko climbing a wall
2. What does it mean that water has a high specific heat and how is
this biologically significant? (think adaptive value)
a. water will change its temperature less when it absorbs or
loses a given amount of heat.
b. large bodies of water keep temp. fluctuations on land
and in water within limits that permit life
c. organisms are made primarily of water, this enables
them to resist changes in their own temp, than if they
were made of a liquid with a lower specific heat.
3. What is the difference between hydrophilic and
hydrophobic?
Examples of Macroelements:
C HOPKNS CaFe Mg NaCl
Control
Without Nitrogen
Goiter – minus Iodine
QUESTIONS FROM CHAPTER 4?
I.
Introduction to Organic Compounds and Their Polymers
A. Carbon
1. Organic molecules – compounds made by cells and contain 2 or more
carbon atoms.
2. 6 electrons…four covalent bonds
3. Hydrocarbons…compounds composed of only carbon and hydrogen
4. Carbon skeleton…chain of carbon atoms in organic molecules
a. may include double bonds
b. vary in length, may be straight, branched, or arranged in rings
c. hydrophobic compounds because bonds between carbon and
hydrogen are nonpolar
Variations in carbon
skeletons.
6. Isomers…compounds with the same molecular formula,
different structures and therefore different properties.
a. Structural Isomers…differ in covalent arrangement of
atoms
b. Geometric isomers…same covalent arrangement,
differ in spatial arrangement
c. Enantiomers or Stereoisomers...molecules that are
mirror images
Structural Isomers
Importance of Functional Groups
B. Functional Groups (pg. 64-65 in book)
1. LEARN to understand properties of organic compounds
2. Attached to carbon skeleton, usually involved in reactions
a. Some are attached to the end
•Hydroxyl group ( -OH) molecules containing this group
are called alcohols
•Amino group (--NH2) molecules containing this group are
called amines.
b. Some include a carbon atom of the skeleton
•Carbonyl group (-C=O) If carbon is at end of skeleton the
compound is called an aldehyde. If carbon if within the chain
it is called a ketone.
•Carboxyl group (-COOH) Compounds in this group are
called carboxylic acids
3. Nonpolar functional groups
a. Sulfhydryl (-SH) Compounds with this group are called
“thiols” Help to stabilize structure of protein.
b. Phosphate (PO4) Anion. Function is the transfer of
energy between molecules (ATP)
C. General terms (this is where chapter 5 starts)
1. Macromolecules
2. Polymer
3. Monomer
4. Dehydration Synthesis (condensation reaction)
5. Hydrolysis
For each Macromolecule know the
following:
•
•
•
•
Elements it contains
Monomer units and structures
Examples
Uses or roles
II. Carbohydrates
A. Monosaccharides
1. Molecular formula CH2O
2. Classifying a sugar
a. hydroxyl group attached to each carbon except for one
b. the one carbon is bonded to an oxygen to form a carbonyl
group (aldose or a ketose depending on location)
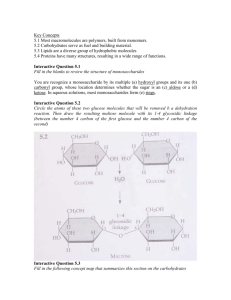
B. Dissacharides
1. “glycosidic linkage” bonds two monosaccharides
2. Sucrose (gl + fr), Maltose (gl + gl), Lactose (gl + ga)
C. Oligosaccharides
1. 2 - 10 joined simple sugars.
2. Used in cell membranes.
D. Polysaccharides
1. polymers of a few hundred to a few thousand
monosaccharides linked together by dehydration
2. Storage molecules
a. starch
b. glycogen
3. building material for protective structures
a. Cellulose
b. Chitin
c. Pectin
III. Lipids
A. Fat or triglyceride
1. nonpolar
2. made of 1 glycerol and 3 fatty acids
a. glycerol is an alcohol
b. fatty acid is a carboxyl group on a hydrocarbon chain
3. Unsaturated vs. Saturated
4. energy storage
B. Phospholipids
1. major component of cell membrane (2007 essay ques)
2. structurally similar to fats, but contain phosphorus and have 2
fatty acids instead of 3
Phospholipids have a hydrophobic tail, but a hydrophilic head.
Self-assembles into bilayers, an important part of cell membranes.
C. Waxes
1. one fatty acid linked to an alcohol
a. more hydrophobic than fats
D. Glycolipid
1. 3rd carbon in glycerol is bonded to a short carbohydrate chain
2. hydrophilic in cell membrane
E. Steroid
1. carbon skeleton is bent to form 4 fused rings (3 six sided rings and
1 five sided ring)
2. Cholesterol is an example
3. female and male sex hormones
4. anabolic steroids (variant of testosterone)
Question ?
• Which has more energy, a kg of fat or a kg of
starch?
• Fat - there are more C-H bonds which provide
more energy per mass.
IV. Proteins
A. Essential to structures and activities of life
1. From Greek word proteios
2. Monomer is amino acid
3. Seven different classes of proteins
a. Structural
b. Contractile
c. Storage
d. Defensive
e. Transport
f. Signal
g. Enzymes
B. Made from 20 kinds of amino acids
1. Basic structure
2. R group determines the properties of each amino acid
C. Amino acids linked by “peptide bonds”
1. Dehydration synthesis
2. Carboxyl group carbon bonds to the amino group nitrogen of the
neighboring amino acid = peptide bond
3. Chain of amino acids = “polypeptide chain”
4. A protein consists of one or more polypeptide chains folded and
coiled into a specific conformation
D. Shape determines its function
E. Denaturation = polypeptide chain unravels, losing shape and thus
function
Amino Acids
Amino Acids
F. There are 4 levels of structure that go into determining the shape of a
protein
1. Primary Structure – amino acid sequence
a. this is what determines the 3-D conformation
2. Secondary Structure – coils and folds made by
polypeptide chain
a. coils caused by hydrogen bonds at regular
intervals along the polypeptide backbone
b. Alpha helix – coil held together by H-bonding
between every 4th amino acid.
c. Pleated Sheet – two regions of the
polypeptide chain lie parallel to each other.
3.Tertiary structure – the overall, 3-D shape of the
polypeptide
a. most are described as “globular” or “fibrous”
b. results from the interactions between the R groups
c. Hydrophobic interaction – type of bonding that causes the
tertiary structure
d. Disulfide bridges – strong covalent bonds that reinforce the
protein conformation. Form when two cysteine monomers are
close together.
4. Quaternary Structure – overall structure that results from the
aggregation of these polypeptide subunits.
a. not all proteins have this structure (only ones with two or
more polypeptide chains.
b. Collagen and Hemoglobin are examples that have this
structure
F. How does a protein know how to fold?
1. Chaperone proteins – molecules that function as temporary
braces in assisting the folding of other proteins.
G. Linus Pauling
Quarternary Structure
Tertiary Structure
Chaperone Protein
(aka “chaperonins”)
A chaperon protein found in E. coli
Provides a
“shelter” for the
folding polypeptid
Primary Structure
Secondary Structure
Tertiary Structure
Quarternary Structure
Is Protein Structure Important?
V. Nucleic Acids
A. Information rich polymers of nucleotides
1. holds recipe to make proteins
2. DNA and RNA
3. a gene codes for one protein (sequence of amino acids)
4. Monomer is a nucleotide (nucleoside monophosphate)
a. a 5-carbon sugar (ribose or deoxyribose)
b. phosphate group (attached to the 5th carbon)
c. nitrogen base (two families – pyrimidines and purines)
Pyrimidines (Cytosine, Thymine, Uracil)
Purines (Guanine, Adenine)
5. DNA is double helix, while RNA is single stranded
6. Phosphodiester linkage creates the phosphate sugar backbone
7. Watson and Crick – Cambridge 1953
Summary
• Role of hydrolysis and dehydration synthesis
• For each macromolecule, know the following:
– Elements and monomers
– Structures
– Functions