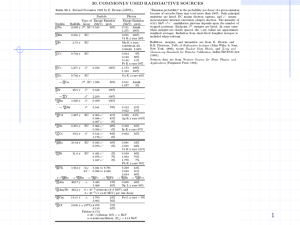
44 Sc - P.Moskal Research Group
advertisement

II International Symposium on Positron Emission Tomography September 21-24, 2014, Jagiellonian University, Kraków, Poland Radiometals for PET diagnostics Renata Mikolajczak NCBJ Radioisotope Centre POLATOM 05-400 Otwock, Poland National Centre for Nuclear Research, Radioisotope Centre POLATOM National Centre for Nuclear Research MARIA Research Reactor National Centre for Nuclear Research, Radioisotope Centre POLATOM Radioisotope Centre POLATOM Division in the National Centre for Nuclear Research Research programs on the development of novel radiopharmaceuticals Results of our research programs and innovation activities can be directly implemented in the GMP certified production and QC facilities. National Centre for Nuclear Research, Radioisotope Centre POLATOM Hot-cells for production of 90Y and 177Lu Radionuclides in medicine National Centre for Nuclear Research Radioisotope Centre POLATOM Radiopharmaceuticals Radiopharmaceutical is a substance formed in a chemical combination of two important components: ligand, chemical compound, molecule or cell which is selectively taken up, metabolized or actively taking part in the physiological process in the imaged organ or tissue. radionuclide, radioactive isotope of certain element – radiation emitted by this isotope is either registered and allows imaging of tracer distribution in the patient’s body or it can destroy the target tissue. 18F-FDG 18F (T1/2 = 110 min) Fludeoxyglucose (18F) injection USP, Ph. Eur. (monograph 1325) Potential targets for molecular imaging 3-integrins CD20, CD22 NIS transcription important target for radiopeptides translation GPCRs m-RNA DNA protein LAT GLUT-1 18FDG extracellular matrix pO2↓ Norep-T [131I]MIBG soluble protein EGFRs MMP Credit to H.R.Maecke pH↓ Principle of Positron Emission Tomography (PET) Wadas TJ et al. Chem Rev 2010;110(5):2858-2902 Schematic Representation of a Drug for Imaging and Targeted Therapy pharmacokinetic modifier Ligand Chelator Linker Target Molecular Address Reporting Unit • Antigens (CD20, HER2) • Antibodies, their fragments and modifications • GPCRs • Regulatory peptides and analogs thereof • 99mTc, 111In, 67Ga • 64Cu, 68Ga • Gd3+ Cytotoxic Unit • Transporters • Amino Acids • • 90Y, 177Lu, 213Bi 105Rh, 67Cu, 186,188Re Credit to H.R. Maecke Tailoring radionuclide half life (energy of emitted radiation) to pharmacokinetics of the ligand The physical half-life of the radionuclide should match with the time required for the radiopharmaceutical to reach the disease/target site and the time required for the radioactivity to be cleared from the body (in vivo residence time) The half-life must be long enough to ensure that the radioactivity of radiopharmaceutical reaching the disease/target localisation will provide the imaging or therapeutic effect. The radionuclide must be attached to the carrier agent in a way that it is stable in vivo so that the radiation is deposited in the desired location. Hence the knowledge of chemical behaviour i.e. the properties of the element itself is important Positron-Emitting Radiometals Isotope T1/2 (h) Methods of Production Decay Mode Eβ+ (keV) 60Cu 0.4 cyclotron, 60Ni(p,n)60Cu β+ (93%) EC (7%) 3920, 3000 2000 61Cu 3.3 cyclotron, 61Ni(p,n)61Cu β+ (62%) EC (38%) 1220, 1150 940, 560 62Cu 0.16 62Zn/62Cu β+ (98%) 2910 generator EC (2%) cyclotron, 64Ni(p,n)64Cu β+ 19(%) EC (41%) β− (40%) 656 9.5 cyclotron, 63Cu(α,nγ)66Ga β+ (56%) EC (44%) 4150, 935 1.1 68Ge/68Ga β+ (90%) EC (10%) 1880, 770 64Cu 12.7 66Ga 68Ga generator 86Y 14.7 cyclotron, 86Sr(p,n)86Y β+ (33%) EC (66%) 2335, 2019 1603, 1248 1043 89Zr 78.5 89Y(p,n)89Zr β+ (22.7%) EC (77%) 897, 909, 1675, 1713, 1744 Wadas TJ et al. Chem Rev 2010;110(5):2858-2902 β- radionuclides suitable for labelling molecules for targeted radiotherapy of tumors (produced in nuclear reactor) Radioisotope 186Re 188Re Half-life Eβ- (max) meV Eγ (%) keV 3.7 d 17 hr 1.07 2.11 137 (9) 155 (15) Production method 185Re(n,γ)186Re 187Re(n, γ) 188Re, 188W/188Re 177Lu generator γ)177Lu, 2.7 d 1.4 d 2.27 0.57, 0.25 1.07 0.69, 0.64 286 (3) 103 (30), 70 (5) 148Nd 153Sm 2.2 d 1.95 d (n,γ)149Nd→149Pm 152Sm(n, γ)153Sm 3 2 166Ho 1.1 d 1.85, 1.77 80 (6), 1379 (1) 164Dy (n, 9 14.3 d 9.6 d 8.0 d 1.71 0.34 0.6 364 (81), 637 (7) 32S(n,p) 32P 0.81 0.57 342 (6) 184 (48), 92 (23) 110Pd 67Cu 7.5 d 2.4 d (n, γ) 111Pd→111Ag 67Zn(n,p)67Cu 2 2 47Sc 3.35d 0.6, 0.44 159 (68) 47Ti(n,p)47Sc, 2 105Rh 149Pm 32P 169Er 131I 111Ag 0.5 3 8 113 (6.4), 208 (11) 319 (19), 306 (5) 90 Y 6.7 d 176Lu Approx. max range in tissue [mm] (n, (n,γ) 177Yb→177Lu 90Sr/90Y generator 104Rn(n,γ)105Rn→105Rh 2 176Yb γ)165Dy (n,γ)166Dy→166Ho 168Er(n,γ)169Er 130Te (n, γ)131Te→131I 12 2 8.2 2 2 46Ca(n,)47Ca→47Sc 199Au 3.2 d 0.46 158 (37), 208 (8) 198Pt(n,γ)199Pt→199Au 2 Matched +/- pairs 44Sc/47Sc 64Cu/67Cu 86Y/90Y 124I/123/131I 47Sc and 67Cu can be produced in nuclear reactor and in cyclotron The „twin” isotope of the same element can be used for diagnostic imaging or therapy follow up, while the other is used for therapy using the same carrier molecules. Matched Radionuclide Pairs for Imaging and Therapy (edited by A. Bockish) Eur J Nucl Med Mol Imaging, Vol 38, Suppl 1, June 2011 Theranostics: combination of diagnosis and therapy Personalized medicine/tailored medicine/ Matching the right drug for the right patient Chelators chelator is used to tightly bind the a radiometal ion so that when injected into a patient, the targeting molecule can be delivered without any radiometal loss Wadas TJ et al. Chem Rev 2010;110(5):2858-2902 DOTA-TATE DOTA-somatostatin analogue Zn2+ 68Ga Fe3+ 177Lu3+ 90Y National Centre for Nuclear Research Radioisotope Centre POLATOM Radiopeptide Therapy in Neuroendocrine Tumors 68Gallium – DOTATATE 90Yttrium – DOTATATE Ga Image Y Treat Credit to H.R. Maecke Ga-68, an innovative radionuclide? GLEASONG,. I. : A PositronCow, Intl. J. Appi.Radiation Isotopes 8:90, 1960. ANGER, H. 0., AND GOTTSCHALK, A. : Localization of Brain Tumors with the Positron Scintillation Camera, J. Nuci. Med. 4:326, 1963. A GALLIUM-68 POSITRON COW FOR MEDICAL USE. YANO Y, ANGER HO. J Nucl Med. 1964 Jun;5:484-7. „To date more than 100 patients have been examined with only a small number of false positives and few known missed tumors.“ Credit to C. Decristoforo 18F 18F + 0.6 110 m cyclotron Organic synthesis and 68Ga for PET 68Ga PET/ CT + 1.9 68.3 m generator coordination chemistry Credit to F. Roesch 68Ge/68Ga generator. 68Ge (p,2n) e 270.8 d 67Ga e; no + 78.3 h 68Ga + 1.9 68.3 m 69Ga 60.1 % 66Zn 27.9 % 6.2 b Credit to F. Roesch Ga-68 DOTATOC vs.SPECT and CT The added value in diagnostic accuracy has an important influence on patient management From ‘onesize sizefits fitsall’ all’toto ‘personalized From ‘one ‘personalized therapy’ therapy’ 18FDG PET-CT before 89Zr-rituximab before 89Zr positron emitter with 72 h half life 18FDG PET-CT 3 months after 90Y-rituximab treatment Courtesy: K. Muylle, P. Flamen, Brussels and G. van Dongen, VUmc, Amsterdam 44Sc and 47Sc 44Sc is a positron emitter (Eβ+ 1475.4 keV with 94.27% positron branching) and gamma radiation component of 1157 keV(99.9%). 47Sc (T1/2 = 3.35 d) is emitting β- radiation with max. energy 0.600 MeV (31.6%) and 0.439 MeV (68.4%) radiation of 159.4 keV (63.3%) suitable for imaging. 44Sc and 47Sc as matched pair for molecular imaging 44Sc Due to the half life (T1/2 = 3.92h) almost 4 times as long as the half life of 68Ga (T 1/2 = 67.71 min) it is an attractive candidate for development of novel PET-radiopharmaceuticals. 47Sc can be utilized in radiotherapy using the same vector molecules Mean range in tissue 47Sc - 810 µm 177Lu – 670 µm 90Y – 3900 µm Both radionuclides can create a matched pair and their clinical application may bring additional value, particularly in combination with ligands requiring longer observation time than in case of 18F or 68Ga labeled molecules. Matched Radionuclide Pairs for Imaging and Therapy (edited by A. Bockish) Eur J Nucl Med Mol Imaging, Vol 38, Suppl 1, June 2011 0.005 M H2C2O4/ 0.07 M HCl 44Ti/44Sc 44Ti + generator 45Ti + 1.9 60.4 a 1.0 3.08 h 43Sc 44Sc + 1.2 3.89 h + 1.5 3.92 h (p,2n) 45Sc 100 % 5 mCi = 185 MBq, elution possible every day in 3 ml volume M. Pruszyński et al., Appl. Radiat. Isot., 68 (2010) 1636 44Ti Production at PSI, Zurich Irradiation of natural scandium 40 16 MeV energy range; up to 50 A current; water jet cooling. 44Ti (p,2n) ε 60.4 a 45Sc 44Ti yield 0.002 – 0.003 MBq/Ah Chemical isolation via anionexchanger 100 % encapsulated target ~2 g scandium Capacity needed for 1GBq of 44Ti equals amount of 68Ge of about 500 000 USD. Konstantin Zhernosekov et al.: Development and evaluation of 44Ti production on high energy protons. P150 19th International Symposium on Radiopharmaceutical Sciences. 28.08-2.09.2011, Amsterdam PSI-Cyclotron Injector II 72 MeV protons Abbas et al. Cyclotron production of 44Sc - new radionuclide for PET technique. 19th International Symposium on Radiopharmaceutical Sciences. 28.08-2.09.2011, Amsterdam Cyclotron production of 44Sc 44Sc – 3,92 h 44mSc 44Ca(p,n)44Sc 44m 44Ca(p,n)44mSc β Sc Sc Ca(stable) 44 44 44Sc 44Sc 0,8 Data for activity calculation intensity – 2 μA 0,7 time – 30 min 0,6 44CaCO 0,5 barn – 58,6 h 0,4 0,3 0,2 0,1 44mSc 0 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 E(MeV) Levkovskij, Act.Cs.By Protons and Alphas, Moscow 1991, USSR 44mSc 3 target – 100 mg Scandium-47 The n.c.a. 47Sc can be produced by proton irradiation in accelerators and in a nuclear reactor. In neutron irradiation there are 2 ways possible: 47Ti(n,p)47Sc 46Ca(n,)47Ca and consecutive -decay of 47Ca both routes require further chemical separation L.F. Mausner, K.L. Kolsky, V.Joshi and S.C.Srivastava.Radionuclide development at BNL for nuclear medicine therapy. Appl Radiat Isot (1998) 49; 285-294 K. L. Kolsky, V. Joshi, L. F. Mausner and S. C. Srivastava. Radiochemical purification of no-carrier-added scandium-47 for radioimmunotherapy Appl Radiat Isot (1998) 49; Chromatographic separation of from 44Ca 44Sc E. Koumarianou et al. 44Sc-DOTA-BN[2-14]NH2 in comparison to 68Ga-DOTA-BN[214]NH2 in pre-clinical investigation. Is 44Sc a potential radionuclide for PET? App Radiat Isot 70 (2012) 2669–2676 Binding affinity study in PC-3 cells 100 %B. rad. [ 125I-Tyr4]-BN DOTA-BN[2-14]NH2 nat 80 Y-DOTA-BN[2-14]NH2 nat Lu-DOTA-BN[2-14]NH2 60 nat Ga-DOTA-BN[2-14]NH2 nat 40 Sc-DOTA-BN[2-14]NH2 IC50 values from displacement study of 125I-[Tyr4]-BN Derivative IC50 (nM) 1.78 ± 0.12 DOTA-BN[2-14]NH2 natY-DOTA-BN[2-14]NH 1.99 ± 0.06 2 natLu-DOTA-BN[2-14]NH 20 natGa-DOTA-BN[2-14]NH natSc-DOTA-BN[2-14]NH 0 -12 2 -11 -10 -9 -8 Log M -7 -6 2 2 1.34 ± 0.11 0.85 ± 0.06 6.49 ± 0.13 -5 Receptor affinity of M-DOTA-BN[2-14]NH2 : Ga>Lu>Y>Sc 44Sc: development of PET-tracers 44Sc-DOTA-TOC PET/CT: 18 h p.i. 44Ti e 44Sc 94 % + 0.60 MeV 3.97 h 44Sc-DOTA-TOC for dosimetric interest and long-term imaging Pruszyński M, Majkowska-Pilip M, Loktionova NS, Rösch F Radiolabeling of DOTATOC with the longer-lived, generator-derived positron emitter 44Sc Pruszyynski M, Loktionova NS, Filosofov DV, Rösch F, Post-elution processing of 44Ti/44Sc generator-derived 44Sc for clinical application Appl Radiat Isot 68 (2010) 1636-1641 Dept. of Nuclear Medicine/P.E.T. Center, Zentralklinik Bad Berka ca 60 a Development of Sc theranostic radiopharmaceuticals C.Müller et al. Promises of cyclotron-produced 44Sc as a diagnostic match for trivalent - emitters: in vitro and in vivo study of a 44ScDOTA-folate conjugate, J.Nucl.Med. 54 (2013) 2168–217 C.Müller et al. Promises of cyclotron-produced 44Sc as a diagnostic match for trivalent - emitters: in vitro and in vivo study of a 44Sc-DOTA-folate conjugate, J.Nucl.Med. 54 (2013) 2168–217 C.Müller et al. Promises of cyclotron-produced 44Sc as a diagnostic match for trivalent - emitters: in vitro and in vivo study of a 44Sc-DOTA-folate conjugate, J.Nucl.Med. 54 (2013) 2168–217 Radioisotopes of Sc with medical potential Radionuclide Reaction Half-life β energies γ energies 47Sc 48Ti(p,2p)47Sc 3.35 d 0.6 (32%) 0.44 (68%) 159 keV (68%) 50Ti(p,2p2n)47Sc 47Ti(n,p)47Sc 46Ca(n,γ)47Sc 44/44mSc 44Ca(d,2n)44Sc 3.97 h / 58.6 h 0.632 MeV 511 keV (94.72%) / 511 keV (100%) 44Sc/44Ti Generator available 3.97 h 0.632 MeV 511 keV (94.72%) 43Sc natTi(p,x) 3.89 h 0.825 (17.2%) 1.198 (70.9%) 511 keV (100%) 47Ti(p,nα) 48Ti(p,2nα) New collaborative project starting : Institute of Nuclear Chemistry and Technology, Heavy Ion Laboratory, UW, POLATOM Diagnostic Radionuclides Positron-Emitters Gamma-Emitters 89Zr, 68Ga, 64Cu, 11C, 13N, 15O, 18F 99mTc,111In, 67Ga, 201Tl, 123I Therapeutic Radionuclides Beta-Emitters Alpha-Emitters 90Y, 186/188Re, 177Lu, 131I, 165Dy, 166Ho, 105Rh, 111Ag 212Bi, 213Bi, 211At, 255Fm, 225Ac Theranostic pairs (matched pairs) 99mTc/ 186/188Re 123/124I/131I 111In/ lanthanides, 90Y 68Ga/67Ga 64Cu/ 67Cu (Auger) Development of multimodality probes for theranostic aplications Gd-complexes Imaging Gd based neutron capture therapy Photodynamic therapy Optical probes MRI probes Theranostics Nuclear probes PET/SPECT for imaging Particle emitters for targeted radionuclide therapy Courtesy H.R. Maecke Developments in Nuclear Medicine Improved access to radionuclides Better understanding of targets Variety of ligands designed for these targets Imaging modalities – improved scanners, hybrid systems SPECT, PET/CT, PET/MR Multidisciplinary approach, collaboration between groups of various expertise – international programs FP6, FP7, COST, EUREKA, IAEA Production of Radiometals at Helmholtz-Zentrum Dresden-Rossendorf, Germany PET-Cyclotron „CYCLONE 18/9” (IBA, Belgium) Cu-61 64Zn(p,a)61Cu labeling of peptides, antibodies (immuno-PET) Cu-64 64Ni(p,n)64Cu labeling of peptides, antibodies, small molecules Y-86 Zr-89 Hg-197(m) 86Sr(p,n)86Y 89Y(p,n)89Zr 197Au(p,n)197(m)Hg dosimetry for antibodies 90Y-labeled peptides, labeling of antibodies (long-term targeting) labeling of peptides, antibodies, small molecules Acknowledgements Helmut R Maecke, Basel Marion de Jong, Rotterdam W.A.P. Breeman, Rotterdam Richard Baum, Bad Berka Alicja Hubalewska-Dydejczyk, Krakow Katarzyna Fröss, Krakow Anna Staszczak, Krakow J aroslaw Cwikla, Warsaw Jolanta Kunikowska, Warsaw Leszek Krolicki, Warsaw Piotr Garnuszek, Warsaw Clemens Decristoforo and Radiopharmacy Committee D. Pawlak, B. Janota, W. Wojdowska, E. Koumarianou Radioisotope Centre POLATOM COST TD1004 – Theragnostics Imaging and Therapy: An Action to Develop Novel Nanosized Systems for Imaging-Guided Drug Delivery (2011-2015), leader of WG1 Imaging reporters for theranostic agents and in COST CM1105 - Functional metal complexes that bind to biomolecules (2012–2016)