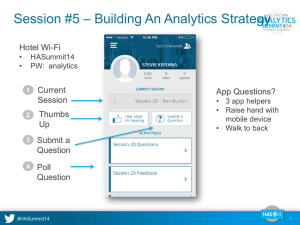
Document
advertisement

Core – Shell anodic catalysts for Direct Methanol and Direct Ethylene Glycol Fuel Cells Dima Kaplan 26.1.11 OUTLINE DMFC and DEGFC DMFC and DEGFC problems Why Core-Shell catalysts? Home made Core-Shell catalysts and their performance Summary 2 What is DMFC? MeOH in CO2 out Anode reaction Cathode reaction Overall reaction CH3OH H 2O CO2 6H 6e 1.5O2 6 H 6e 3H 2O E˚a = 0.04 Volt vs. SHE E˚c = 1.23 Volt vs. SHE CH 3OH 1.5O2 CO2 2 H 2O E˚cell = E˚c – E˚a = 1.19 Volt 3 What is DEGFC? EG in CO2 out Anode reaction CH 2OH 2H 2O 2CO2 10H 10e E˚a = 0.01 Volt vs. SHE 2 Cathode reaction 2.5O2 10 H 10e 5H 2O E˚c = 1.23 Volt vs. SHE Overall reaction CH 2OH 2 2.5O2 2CO2 3H 2O E˚cell = E˚c – E˚a = 1.22 Volt 4 DMFC: current and possible applications Civilian applications SFC EFOY – works like a mobile charger for the car’s battery. Toshiba Dynario – Allows charging of Mobile Electronic Devices via a USB cable. Military applications SFC Emily – recharges batteries that power the electrical devices on board the vehicle (radios, GPS, onboard computers) while the engine isn’t running. SFC JENNY 600S – man portable FC, can power a number of electrical devices such as digital communications and navigation systems, computer and laser tracking devices, remote sensors, cameras and metering devices 5 Catalysts for DAFC • Currently, PtRu alloys are the most apropriate catalysts for DMFC. • For operational temperature of 60°C – 80°C an alloy with atomic ratio of 1:1 was found to be most suitable for DMFC. • Pt is responsible for MeOH and EG dehydrogenation, while Ru is responsible for H2O breakup, thus enabling the formation of CO2 at an acceptable potentials 6 Problems preventing wide spread usage of DMFC • Platinum is used as catalyst on both electrodes. Currently, fuel cells use high Pt loadings, which leads to high catalyst cost. • Nafion is used as the PEM. However, nafion is also expensive. • Methanol crossover trough the PEM leads to reduction of efficiency • Long term durability is questionable due to: – Anode catalyst poisoning by oxidation intermediates and loss of structure integrity – Cathode catalyst poisoning by methanol crossover, surface oxide formation and loss of hydrophobic properties 7 EG as potential fuel for DAFC Pros: • Higher boiling point (1980C vs. 64.70C ) • Lower toxicity than methanol • Greater volumetric capacity (4.8Ah/ml vs. 4.0Ah/ml) • Larger molecule, fuel crossover to the cathode can be much lower Cons • Lower gravimetric capacity (4.32Ah/g vs. 5Ah/gr) • Complicated oxidation mechanism, high number of intermediates • Current anode catalysts are optimized for methanol oxidation 8 So what about the catalyst’s cost…..? 9 Proposed solution Core – Shell catalysts: Pt only in the shell Because the catalysis occurs only on the surface of the electrode it’s logical to use Pt only in the shell of the nano-particals Since exposed Ru sites are needed to break down H2O molecules, a partial monolayer of Pt on top of Ru core should be used PtRu shell Ru core It’s likely, that the best surface PtRu composition for methanol oxidation will be atomic ~1-3:1 depending on the electro-oxidation mechanism EG oxidation might require a different surface composition 10 MA1 catalyst Pt on Ru on XC72 MA1 catalyst was prepared in a two stage synthesis: 1. Electroless deposition of Ru on XC72, using EG as reducing agent 2. Electroless deposition of Pt on Ru/XC72, using NaBH4 as reducing agent MA1a catalyst - composition Metal XPS results Surface atomic ratio EDS results Weight ratio Ru 1 55 Pt 4.28 45 XRD particle size 5.4 nm for Ru 2.7 nm for Pt Comparison to JM HiSPEC 7000: Pt:Ru (1:1) alloy catalyst with carbon support, 45% TM 11 MA1 catalyst – MeOH oxidation activity Catalytic activity - MeOH oxidation ECSA Metal MeOH oxidation 0.5 H2SO4 + 0.1M MeOH 1200 800 -1 Ma [amp*gr Pt] 1000 MA1 catalyst I0.45V [m2/gr PtRu] [A/gr Pt] [A/gr PtRu] [A/m2 PtRu] MA1 29 471 214 7.37 JM HiSPEC 7000 27 346 230 8.52 JM HiSPEC 7000 600 400 200 0 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 V Vs SHE [volt] 0.9 1.0 1.1 1.2 12 MA1 catalyst – EG oxidation activity Catalytic activity - EG oxidation ECSA Metal 2500 2250 -1 Ma [amp*gr Pt] 2000 EG oxidation MA1 catalyst JM JMHiSPEC HiSPEC7000 7000 0.5M H2SO4 + 0.4M EG I0.45V [m2/gr PtRu] [A/gr Pt] [A/gr PtRu] [A/m2 PtRu] MA1 29 241 109 3.75 JM HiSPEC 7000 27 263 175 6.50 1750 1500 1250 1000 750 500 250 0 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 V Vs SHE [volt] 0.9 1.0 1.1 1.2 13 DK6a catalyst Pt on Ru on XC72 DK6a catalyst was prepared in a two stage successive deposition synthesis: 1. Electroless deposition of Ru on XC72, using NaBH4 as reducing agent 2. Electroless deposition of Pt on Ru/XC72, using NaBH4 as reducing agent DK6a catalyst - composition Metal XPS results Surface atomic ratio EDS results Weight ratio Ru 1 80 Pt 0.47 20 XRD particle size 1.3 nm 14 DK4a catalyst PtRu on IrNi on XC72 DK4a catalyst was prepared in a two stage successive deposition synthesis: 1. Electroless deposition of IrNi on XC72, using NaBH4 as reducing agent 2. Electroless deposition of PtRu on IrNi/XC72, using NaBH4 as reducing agent DK4a catalyst - composition Metal XPS results Surface atomic ratio EDS results Weight ratio Ru 1 11 Pt 0.33 19 Ir 0.28 69 XRD particle size 2 nm 15 MeOH oxidation activity summary Catalyst Surface composition (XPS) MA1 20%Pt/24%Ru/XC72 Ma Ma ECSA Sa ]amp/gr Pt] ]amp/gr TM] Ru:Pt 1:4.28 471 214 29 7.37 DK6a 15%Pt/59%Ru/XC72 Ru:Pt 1:0.47 204 41 29 1.40 DK4a 22%PtRu/54%IrNi/XC72 Ru:Pt:Ir 1:0.33:028 920 101 25 4.04 JM HiSPEC 7000 45%PtRu/carbon Ru:Pt 1:1.67 346 230 27 8.52 JM HiSPEC 12100 75%PtRu/carbon Ru:Pt 1:1.9 620 416 50 8.32 2 ]m /gr TM[ ]amp/m2 TM[ JM HiSPEC 12100: PtRu (1:1) alloy catalyst with carbon support, 75% TM 16 EG oxidation activity summary Catalyst surface composition (XPS) MA1 20%Pt/24%Ru/XC72 Ma Ma ECSA Sa [amp/gr Pt] ]amp/gr TM] Ru:Pt 1:4.28 241 109 29 3.75 DK6a 15%Pt/59%Ru/XC72 Ru:Pt 1:0.47 526 105 29 3.62 DK4a 22%PtRu/54%IrNi/XC72 Ru:Pt:Ir 1:0.33:028 341 37 25 1.48 JM HiSPEC 7000 45%PtRu/carbon Ru:Pt 1:1.67 263 175 27 6.50 JM HiSPEC 12100 75%PtRu/carbon Ru:Pt 1:1.9 316 212 50 4.24 2 ]m /gr TM] ]amp/m2 TM] 17 Summary Several core – shell catalysts were synthesized. One of them (DK4a) showed a superior performance in methanol oxidation over commercial HiSPEC 12100 catalyst. The other catalyst (DK6a) showed a superior performance in EG oxidation over commercial HiSPEC 12100 catalyst. Core shell catalysts have a potential to drastically reduce the Pt loadings currently needed in DMFC and DEGFC. Efforts to find a durable and cheaper (than Ru) core metal should be made. The results show that EG and methanol might require different surface compositions of Pt:Ru. 18 Acknowledgments • • • • • Prof. Emanuel Peled Dr. Larisa Burstein Dr. Yuri Rosenberg Dr. Jack Penciner All the electrochemistry group of TAU 19