Stoichiometry Practice Problems: Mole Conversions & Calculations
advertisement
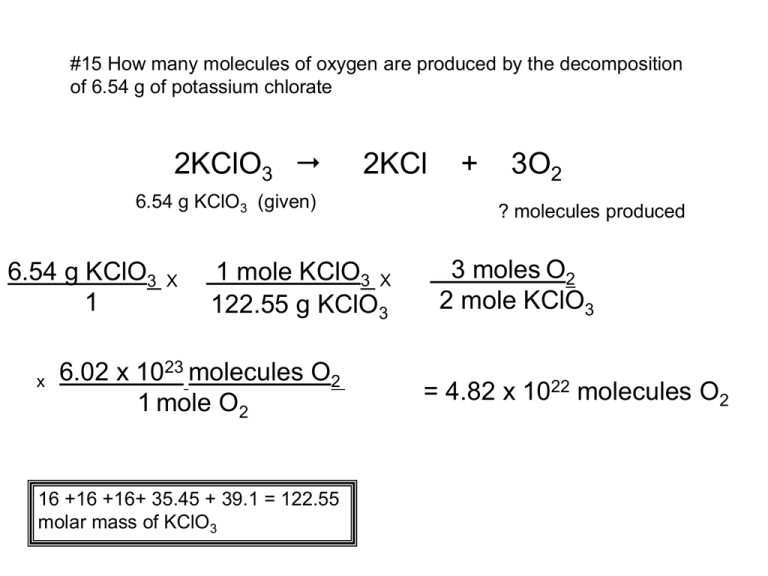
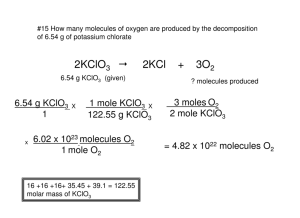
#15 How many molecules of oxygen are produced by the decomposition of 6.54 g of potassium chlorate 2KClO3 2KCl 6.54 g KClO3 (given) 6.54 g KClO3 1 x X 1 mole KClO3 X 122.55 g KClO3 6.02 x 1023 molecules O2 1 mole O2 16 +16 +16+ 35.45 + 39.1 = 122.55 molar mass of KClO3 + 3O2 ? molecules produced 3 moles O2 2 mole KClO3 = 4.82 x 1022 molecules O2 #16 The last step in the production of nitric acid is the reaction of nitrogen dioxide with water. 3NO2 + H2O ? g NO2 2HNO3 + NO 5.00 x 1022 molecules NO (given) 1 mole NO x 5.00 x 1022 molecules NO x 1 6.02 x 1023 molecules NO 3 moles NO2 x 1 mole NO 16 +16 +14 = 46 molar mass of NO2 46 g NO2 1 mole NO2 = 11.5 g NO2 #17 The equation for the combustion of carbon monoxide is: 2CO + O2 2CO2 How many liters of Oxygen are required to burn 3.86 L of carbon monoxide? 2CO + O2 3.86 L CO 2CO2 ? L O2 3.86 L CO x 1 mole CO x 1 mole O2 x 22.4 L O2 22.4 L CO 2 mole CO 1 mole O2 1 = 1.93 L O2 #18 Phosphorus and hydrogen can be combined to form phosphine (PH3) 6H2(g) 4PH3(g) P4(s) + How many liters of phosphine are formed when 0.42 L of hydrogen reacts with phosphorus? P4(s) + 6H2(g) 4PH3(g) ? L PH3 0.42 L H2 0.42 L H2 1 x 1 mole H2 22.4 L H2 22.4 L PH3 1 mole PH2 x 4 moles PH3 6 moles H2 = 0.28 L PH3 x #19 Consider this equation: CS2 3O2 CO2 + 2SO2 + 27.9 mL O2 ? L SO2 (the only trick here is to convert to liters from mL and the rest of the problem is the same) 27.9 mL O2 1 1 L O2 x 1 mole O2 x 1000 mL O2 22.4 L O2 x 2 moles SO2 3 moles O2 x 22.4 L SO2 1mole SO2 = .018 L SO2 18.6 mL SO2 #20 Consider this equation: CS2 + 3O2 CO2 + 2SO2 ? dL CO2 0.38 L SO2 (the only trick here is to convert to deciliters from Liters) 1 dL is 100 ml or 1/10 of a liter 0.38 L SO2 1 x 22.4 L CO2 x 1 moles CO2 1mole SO2 22.4 L SO2 10 dL CO2 1 L CO2 x 1 mole CO2 x 2 moles SO2 = 1.9 dL CO2 .19 L CO2