HomeworkThree
advertisement

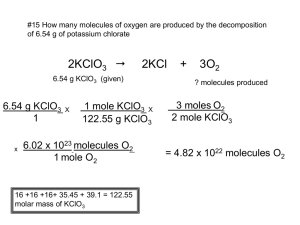
Mary Anne Rooney Hmwk 3 - Page 1 MISE - Physical Basis of Chemistry Third Set of Problems - Due November 26, 2006 Submit electronically (digital drop box) by Sunday, November 26 – by 6 pm. Note: When submitting to digital Drop Box label your files with your name first and then the label HmwkThree. Please PUT YOUR NAME in the ‘HEADER’ along with the alreadyinserted page #. The following information may be useful when solving the problems: Ideal gas law: PV = nRT ; where R = 0.0821 L•atm•mole-1•K-1. 1 mole = 6.022 x 1023 “things”. The atomic weight of an element listed in the periodic table is the mass of one mole of atoms in grams. This is termed the molar mass (i.e., mass per mole) of the element. The molecular weight - determined from molecular formula as the sum of the atomic weights of all of the constituent atoms - is the mass of one mole of molecules in grams. This is termed the molar mass (i.e., mass per mole) of the molecule. 1 atmosphere (atm) = 760 mm Hg = 76 cm Hg = 101.3 kilopascals. 1000 cm3 = 1 L ; K = ºC + 273 . 1. Given: 3 identical containers (same volume), at 1 atm pressure and 0 ºC. Each flask is filled with a different gas. Flask A: CO Flask B: NO2 Flask C: He Answer each of the following and briefly explain your answer using the concepts covered in class: (a) Which flask contains the greatest number of moles of gas? Each flask contains one mole of gas because the volume is the same at standard temperature and pressure. (b) Which flask contains the largest total mass of gas? The B flask containing NO2 has the greatest total mass because the molecular weight of the particles of gas per mole is the greatest for NO2. Flask A has a total mass per mole of 28 g. Flask B has a total mass per mole of 46 g. Flask C has a total mass per mole of 4 g. (c) In which flask will the gas molecules have the greatest average kinetic energy? All three flasks of gases will have the same average kinetic energy because each flask contains one mole of gas at the same volume, temperature, and pressure. The formula used to calculate average kinetic energy is (3RT)/2; therefore, the average kinetic energy would be (3 x 8.314 J/mol*K x 273 K)/2 in all three flasks. (d) In which flask will the molecules have the greatest average velocity? The C flask containing He has the greatest average velocity because the average velocity in inversely proportional to the mass of the gas. The formula used to calculate average velocity is √(8RT)/πM; therefore, uavgCO = √((8 x 8.314 x 273)/π x 0.028 = 454.3 m/s uavgNO2 = √((8 x 8.314 x 273)/π x 0.046 = 354.5 m/s uavgHe = √((8 x 8.314 x 273)/π x 0.004 = 1202.1 m/s Mary Anne Rooney Hmwk 3 - Page 2 2. A mixture of gases effusing … A chamber is filled with a gaseous mixture consisting of 1.00 mole of He gas and 4.00 moles of SO2 gas at a certain temperature. [The atomic weight of He is 4.00 g/mole and the molecular weight of SO2 is 64.0 g/mole] The mixture is allowed to escape into a series of two evacuated chambers (“1” & “2”) connected by pinholes. Please refer to the diagram below. 1 2 Initial Chamber You may assume that all three chambers have the same volume and are rigid. You may also assume that both pinholes are exactly the same area. Finally, temperature is constant throughout the entire process. [Recall the kinetic theory lecture slides (especially #15) depicting the process and relevant relationship to determine how the composition of a mixture of gases changes when the mixture effuses (leaks) through a pinhole into an evacuated chamber.] A useful proportion when working in moles is the mole percent. If we have a mixture of substances, “A” and “B”, the mole % is defined as: moles A moles A mole % A = • 100 % = • 100 % moles A + moles B total moles mole % B = moles B moles B • 100 % = • 100 % moles A + moles B total moles Of course, mole % A + mole % B = 100 %. Now we are ready to go. Please show your work. (a) Determine the mole percent of each gas in the initial chamber before effusion occurs. To determine the mole percent of each gas we need to use the mole ratio which was presented in the problem. He = (1 mole/5 total moles) * 100 = 20% and SO2 = (4 moles/5 total moles) * 100 = 80% (b) Determine the ratio of moles He: moles SO2 in chamber 1 a few moments after the gaseous mixture begins to effuse through the pinhole into evacuated chamber 1. Now, determine the mole percent of each gas in this effused mixture. Zeffusion = (#moleculesHe/ΔtimeHe) = (A/4 x NHe/V x √(8RT)/(π x MHe) (#moleculesSO2/ΔtimeSO2) (A/4 x NSO2/V x √(8RT)/(π x MSO2) By canceling before we substitute numbers, we have simplified the equation for anything that is the same for both gases; therefore: Zeffusion = # molecules He = N He x √(M SO2/M He) # molecules SO2 N SO2 (Based on 100 molecules of mixture): 20He / 80SO2 = (1 molHe / 4 molSO2) x √(64.0 g/molSO2)/(4.00 g/molHe) = (1/4) x √16 =¼x4 = 1 which is written as the ratio 1:1 Mary Anne Rooney Hmwk 3 - Page 3 or 1 particle He to 1 particle SO2; therefore, The mole percent of Helium is 1:2 x 100 = 50% Helium and the mole percent of SO2 is also 1:2 x 100 = 50% SO2 after a few minutes of effusion. (c) Determine the ratio of moles He : moles SO2 in chamber 2 a few moments after the gaseous mixture begins to effuse through the pinhole into evacuated chamber 2. Now, determine the mole percent of each gas in this effused mixture. Using the same formula from the previous section of this problem, we can calculate the effused mixture again but with the new mole percents from the first chamber. Zeffusion = # molecules He = N He x √(M SO2/M He) # molecules SO2 N SO2 = (1 molHe/ 1 molSO2) x √(64.0 g/mol)/(4.00 g/mol) = (1/1) x √16 =1x4 = 4 which is written as the ratio 4:1 (He/SO2) so The mole percent of He is 4:5 x 100 = 80% He and the mole percent of SO2 is 1:5 x 100 = 20 % SO2 after a few minutes of effusion. (d) In class, we said that the effusion process creates a gaseous mixture that is enriched in the gas of lower molar mass. Thus, if more than one effusion occurs, each newly effused mixture is even more enriched in the gas of lower molar mass. Compare your results to (a), (b), and (c) and briefly comment on the validity of these assertions. The validity of these assertions can be seen in the following chart: mole % He mole % SO2 initial 20 80 first effusion 50 50 second effusion 80 20 and in addition to this chart, the problem stated that the molar mass of SO2 was 64.0 g/mol and the molar mass of He was 4.00 g/mol. 3. “Real” gases - relaxing some of the postulates from kinetic theory. Recall the postulates of the kinetic theory of gases (see kinetic theory lecture slide #2). These postulates rationalize the ideal gas law (PV = nRT) at the molecular level. The two postulates that may be the most “unrealistic” are the first two: 1. Gas particles have no volume, i.e., they are point particles. 2. There are no inter-particle forces - neither attractive nor repulsive. A more “realistic” model of gas behavior modifies each of the above postulates. The two modified postulates are: 1. Gas particles have volume. If we presume that the particles are “hard spheres”- like “micro” billiard balls - they will occupy some non-negligible volume of the container volume. Thus, the “empty space”, termed, the available volume, is necessarily less than the full volume of the container. So, if V is the volume of the container, the volume available to the gas particles is V minus the unavailable volume due to the spheres. In brief, due to particle size, there is less space available to the gas molecules than the full container volume would imply. 2. There are inter-particle (usually termed intermolecular) forces between the particles. The most important are attractive forces. Thus, gas particles “feel each other’s presence” and “pull” on each other. We may thus imagine that a molecule in the “middle” of the sample - if evenly surrounded by its identical neighbors - may not feel any pull at all due to the balancing forces, as you learned in physics. However, a Mary Anne Rooney Hmwk 3 - Page 4 molecule “next to the wall” only has neighbors beside it and behind. Now the forces will be unbalanced and so there may be some net pull. So, a molecule approaching the wall may hit with less force (why?) and/or be diverted from hitting the wall at all. Let’s now consider how the pressure of the gas - as predicted by the ideal gas law - is altered by the two modified postulates above. Of course, pressure is still force/area - this is its definition. We will continue to presume that the microscopic origin of the macroscopic gas pressure is due to the molecules colliding with the container walls. (a) How would the pressure (P) of the gas be altered from its ideal value (Pideal), due only to modified postulate 1? [The ideal pressure (= nRT/V) is what we would expect if the particles followed the original postulates from kinetic theory.] Please select one of the choices below and then carefully explain your choice. No calculations required or expected. P < Pideal P > Pideal P = Pideal (no change) If the particles have a larger size than their volume will be larger which means that the space available to them will be less. Boyles Law states that less volume means more pressure so therefore P > Pideal (b) How would the pressure (P) of the gas be altered from its ideal value (Pideal), due only to modified postulate 2? [The ideal pressure (= nRT/V) is what we would expect if the particles followed the original postulates from kinetic theory.] Please select one of the choices below and then carefully explain your choice. No calculations required or expected. P < Pideal P > Pideal P = Pideal (no change) Attraction would decrease the number of collisions. Since it is the collisions that are the pressure, when there are attractions there would be fewer and less frequent collisions; therefore, P < Pideal (3c) Now imagine two “real” gases “X” and “Y”, where each gas is presumed to fulfill both of the modified postulates (molecules have volume and experience intermolecular attractive forces). Gases X and Y are in separate containers of the same volume at the same temperature. Each container contains the same number of moles of gas. A measurement of the pressure of gas X at these conditions is 15.0 atm. A measurement of the pressure of gas Y at these conditions is 11.0 atm. Please answer the following and explain you answers. No calculations required or expected. (i) According to the ideal gas law, Pideal for each gas should be the same. Why? They should be the same because they would only depend upon how many particles there are the # of moles would be inversely proportional to the MW. (ii) The calculated ideal pressure is 12.0 atm. Considering each gas separately, which “non-ideal” factor (molecular volume or attractive forces) is contributing most to the measured pressure? How do you know? Since the pressure of gas X increased from the ideal, the size of the particles would be larger based on modified postulate 1. With gas Y more intermolecular attractions would explain the decrease in pressure based on modified postulate 2