Practice Problems Ch. 9.2
advertisement

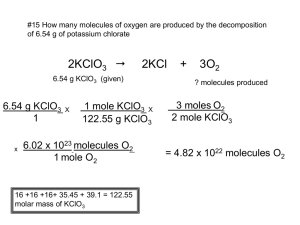
Email: Msoconnor99@gmail.com Heferguson@wascohsd.org Practice Problems Ch. 9.2 Ratios are found using the coefficients. The coefficient represent molecules and moles of that substance in the equation. You are applying what you already know with the mole train and using it combined with mole ratios! Pg. 244 #9 This equation shows the formation of aluminum oxide: 4Al + 3O2 2Al2O3 a. Write the six mole ratios that can be derived from this equation. i. 4:3 and 3:4 (Al:O2 and O2:Al) ii. 4:2 and 2:4 (Al: Al2O3 and Al2O3:Al) iii. 3:2 and 2:3 (O2: Al2O3 and Al2O3: O2) b. How many moles of aluminum are needed to form 3.7mol Al2O3? i. Simple mole to mole conversion ii. Begin with what have: 3.7mol Al2O3 iii. Determine what you want: mol of Aluminum (Al) iv. Ratio will be wanted/given: 4/2 (Al/Al2O3) (Coefficients from the balanced equation) v. 3.7mol Al2O3 x 4mol Al/2mol Al2O3 = 7.4mol Al vi. 3.7mol Al2O3 x 4mol Al/2mol Al2O3 = 7.4mol Al (units will cancel out) Pg. 244 #10 According to the equation in problem 9: a. How many moles of oxygen are required to react completely with 14.8mol Al? i. Simple mole to mole conversion ii. Begin with what you have: 14.8 mol Al iii. Determine what you want: mol Oxygen (O2) iv. Ratio will be wanted coefficient/given coefficient: 3/4 (O2/Al) v. 14.8mol Al x 3mol O2/4mol Al = 11.1mol O2 vi. 14.8mol Al x 3mol O2/4mol Al = 11.1mol O2 b. How many moles of Al2O3 are formed when 0.78mol O2 reacts with aluminum? i. Simple mole to mole conversion ii. Begin with what you have: 0.78 mol of O2 iii. Determine what you want: Mol Al2O3 iv. Ratio will be wanted coefficient over given coefficient: 2/3 (Al2O3/O2) v. 0.78mol O2 x 2mol Al2O3/3mol O2 = 0.52mol Al2O3 vi. 0.78mol O2 x 2mol Al2O3/3mol O2 = 0.52mol Al2O3 Pg. 248 #13 How many molecules of oxygen are produced by the decomposition of 6.54g of potassium chlorate (KClO3)? 2 KClO3 2KCl + 3O2 Email: Msoconnor99@gmail.com Heferguson@wascohsd.org Complex conversion. Convert to moles, use mole ratio, convert to molecules Begin with what you have: 6.54g KClO3 Determine what you want: molecules of oxygen (O2) Ratio will be wanted coefficient over given coefficient: 3/2 (KClO3/O2) 6.54g KClO3 x (1mol KClO3/ 122g KClO3) x 3mol O2/2mol KClO3 x 6.02 x1023 molecules O2/1mol O2 = 4.84 x1022molecules O2 Let’s take it step by step o Convert 6.54g KClO3 to moles: 6.54g KClO3 x 1mol KClO3/ 122g KClO3 = .054mol KClO3 o Apply mole ratio to convert to O2: .054mol KClO3 x 3mol O2/2mol KClO3= .081mol O2 o Convert to molecules of O2: .081mol x 6.02 x1023 molecules O2/1mol O2 = 4.84x1022molecules O2 Pg. 248 #14 The last step in the production of nitric acid is the reaction of nitrogen dioxide with water: 3NO2 + H2O 2HNO3 + NO How many grams of nitrogen dioxide must react with water to produce 5.00 x 1022 molecules of nitrogen monoxide? Complex conversion. Convert to moles, use mole ration, and convert to grams. Begin with what you have: 5.00 x 1022 molecules nitrogen monoxide (NO) Determine what you want: Grams of nitrogen dioxide (NO2) Ratio will be wanted coefficient over given coefficient: 3/1 (NO2/NO) Step by step o Convert to moles: 5.00 x 1022 molecules NO x 1mol NO/6.02 x1023 molecules NO = 0.083mol NO o Apply Mole ratio to convert to NO2: 0.083mol NO x 3mol NO2/1mol NO = 0.249mol NO2 o Convert to grams of NO2: 0.249mol NO2 x 46g NO2/1mol NO2 = 11.45g NO2 Show all your work when solving the problems because we are not masters yet! You can easily find your mistake if you show your work. Just take it step by step. Apply your knowledge of the mole train and your new knowledge of mole ratios. Most people don’t get this down in one try, that’s okay. Keep practicing and it will become like second nature to you.