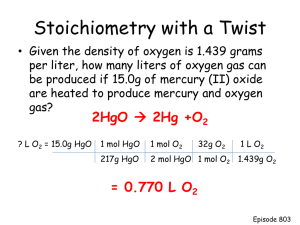Bi 2 O 3
advertisement

Motivation of research work Recent research efforts have focused on its high density and even pure BiFeO3 and research work has not enough achievement. In the present work, we report on the effects of making glass and crystalize BFO in glasses. Purpose of a research talk 1. To research about recent research efforts of Bismuth-Ferrite (BiFeO3 ) 2. To determine optimal parameters of being Bismuth-Ferrite • To determine to depending on glass’s amorphous from Bi2O3 and Fe2O3 components • To determine critical temperature of annealing in BFO in silica glasses Bismuth-Ferrite Bismuth Ferrite (BiFeO3) is perhaps the only material that is both magnetic and a strong ferroelectric at room temperature. Bismuth ferrite (BiFeO3) is an inorganic chemical compound with a perovskite structure. It is one of the most promising lead-free piezoelectric materials by exhibiting multiferroic properties at room temperature. Multiferroic materials exhibit ferroelectric or antiferroelectric properties in combination with ferromagnetic (or antiferromagnetic) properties in the same phase. Compositional phase diagram of Bi2O3 and Fe2O3 Bismuth Ferrite is usually prepared from equal parts of Bi2O3 and Fe2O3. Phase diagram of Bi2O3 and Fe2O3 shows it. Technology of extracting glass ceramic BFO Bi2O3 Fe2O3 SiO2 Mix powders Melting at 11500С, 1 hour Casting and glass formed Annealing Glass ceramic BiFeO3 K2CO3 Bismuth Ferrite (BiFe03) Typical Applications 1) Use in new high tech magnetic tapes 2) Superconductivity 3) Environmental engineering 4) To enhance spontaneous magnetization Theoretical process? Glass is metastable and will transform to the stable crystalline state if enough thermal energy is available. This transformation is called devitrification or crystallization, and occurs by a two-step nucleation and crystal growth process. When the temperature is increased high, crystal nuclei begin to form. Crystallization makes glass opaque and does improve its other properties such as strength and hardness. Glass Heat treatment/annealing/ Glass ceramic Controlled or Uncontrolled crystallization? Controlled crystallization Criteria of controlled crystallization are: High nucleation frequency, uniform throughout the entire glass volume. Very uniform crystal size Very small crystallite dimensions(usually only a few micrometers) Uncontrolled crystallization Uncontrolled crystallization is one kind of defect. Experimental procedure-I (Melt a glass) To prepare the BFO, molar percent of Bi2O3 and Fe2O3 should be equal. This precursor was melted at 11500C by four versions: 25:25, 20:20, 15:15, 10:10. Bi2O3 (mole %) Fe2O3 (mole %) Glass-J 25 25 Glass-G 20 20 Glass-I 15 15 Glass-H 10 10 Composition and raw materials calculation (for glass J) Raw materials g/mol 100g batch raw materials Bi2O3 465.93 60.6 Fe2O3 159.65 SiO2 60.07 K2CO3 138.18 M [g/mol] Mol % Weight in g Weight % 100g batch Bi2O3 465.93 25 116.48 60.6 60.6 Fe2O3 159.65 25 39.9 20.8 20.8 SiO2 60.07 33.33 20.02 10.42 10.42 K2O 94.19 16.67 15.7 8.2 8.2 20.8 10.4 12.054 XRD patterns of glasses Fig. shows XRD patterns of glasses melted at 11500C by four versions: 25:25, 20:20, 15:15, 10:10 for 1 hour. One of glasses was defect, it has uncontrolled crystallization(glass-J). Other 3 glasses were amorphous Study of Atomic force microscope: Bi2O3:Fe2O3=20:20 Glass-G’s surface nanostructure, AFM, it was not uniform totally. Appearance of Glass-G (20:20) Study of Atomic force microscope: Bi2O3:Fe2O3=15:15 Glass-I’s surface nanostructure, AFM, it was uniform totally. Appearance of Glass-I (15:15) Study of Atomic force microscope: Bi2O3:Fe2O3=10:10 Glass-H’s surface nanostructure, AFM, it wasn’t uniform totally. Appearance of Glass-H (10:10) Experimental procedure-II (Annealing of glasses) After melting, we have annealed glasses by below condition. When we choose annealing temperature 4000С and 5000С , based on pre research work. Annealing condition, results Glasses Annealing temperature, 0C Annealing time, h Results Glass-J,G,I,H 4000C 6 Crystallized Glass-J,G,I,H 5000C 6 Crystallized Annealing at 5000C Fig. shows XRD patterns of BFO annealed at 5000C for 6 hours. The BFO was decomposed to Bi2O3 and Fe2O3, it seems that temperature was high and not convenient to crystallize BFO. Annealing at 4000C (Bi2O3:Fe2O3=20:20) Fig. shows XRD patterns of BFO annealed at 4000C for 6 hours. The BFO contained nonperovskite phase, such as Bi2Fe4O9, Bi2.88Fe5O12 , red is BiFeO3. Annealing at 4000C (Bi2O3:Fe2O3=15:15) Fig. shows XRD patterns of BFO annealed at 4000C for 6 hours. The BFO contained nonperovskite phase, such as Bi2Fe4O9, Bi2.88Fe5O12 , red is BiFeO3. Annealed samples Bi2O3:Fe2O3=20:20 Bi2O3:Fe2O3=15:15 Conclusions Glass-I was the most amorphous glass and glass-J was uncontrolled crystallization. All glasses crystallized at 4000C, annealing temperature 5000C was inappropriate to glass ceramic BFO. BFO started to form at 4000C and time was short, so should be increase annealing time. In recent work, we used Bi2O3 : Fe2O3 which were not high purity, that’s why BFO contained non-perovskite phase. References Sven Bossuyt, California Institute of Technology Pasadena, California 2001 Peculiarities of a Solid-state synthesis of Multiferroic Polycrystalline BiFeO3, Matjaz Valant, Anna-Karin Axelsson and Neil Alford, 2007 Materials Letter, 2008 Effects of Annealing Atmosphere on Crystallization and Electrical Properties in BiFeO3 thin films by chemical solution deposition. Kwi-Young YUN, Minoru Noda and Masanori Okuyama, 2003 ‘Microstructure of glass-ceramics and photosensitive glasses ’ Wiss. Ztschr.Friedrich-Schiller-University, Jena1979 Thanks for pay attention!